Novel 8-Heterocyle Substituted Tetracyclines are Potent and Broad
advertisement
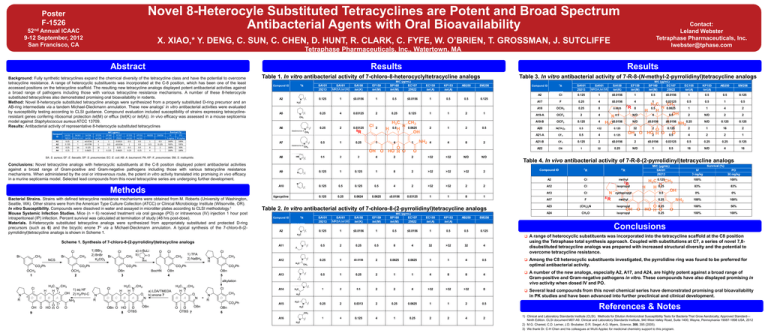
Novel 8-Heterocyle Substituted Tetracyclines are Potent and Broad Spectrum Antibacterial Agents with Oral Bioavailability Poster F-1526 52nd Annual ICAAC 9-12 September, 2012 San Francisco, CA X. XIAO,* Y. DENG, C. SUN, C. CHEN, D. HUNT, R. CLARK, C. FYFE, W. O’BRIEN, T. GROSSMAN, J. SUTCLIFFE Tetraphase Pharmaceuticals, Inc., Watertown, MA Abstract Methods Results Background: Fully synthetic tetracyclines expand the chemical diversity of the tetracycline class and have the potential to overcome tetracycline resistance. A range of heterocyclic substituents was incorporated at the C-8 position, which has been one of the least accessed positions on the tetracycline scaffold. The resulting new tetracycline analogs displayed potent antibacterial activities against a broad range of pathogens including those with various tetracycline resistance mechanisms. A number of these 8-heterocycle substituted tetracyclines also demonstrated promising oral bioavailability in rodents. Method: Novel 8-heterocycle substituted tetracycline analogs were synthesized from a properly substituted D-ring precursor and an AB-ring intermediate via a tandem Michael-Dieckmann annulation. These new analogs’ in vitro antibacterial activities were evaluated by susceptibility testing according to CLSI guidance. Compound evaluation included susceptibility of strains expressing tetracyclineresistant genes confering ribosomal protection tet(M) or efflux (tet(K) or tet(A)). In vivo efficacy was assessed in a mouse septicemia model against Staphylococcus aureus ATCC 13709. Results: Antibacterial activity of representative 8-heterocycle substituted tetracyclines Compund ID A1 A2 A3 A4 SA101 29213 0.25 M RSA ,tet (M ) 4 SA158 tet (K) ≤0.0156 0.125 1 ≤0.0156 0.25 0.25 16 2 0.125 0.0625 EF159 tet (M) 4 1 16 2 SP160 tet (M) 1 0.5 1 0.125 EC107 25922 0.0313 ≤0.0156 0.25 0.0625 Results Results Table 1. In vitro antibacterial activity of 7-chloro-8-heterocyclyltetracycline analogs Table 3. In vitro antibacterial activity of 7-R-8-(N-methyl-2-pyrrolidinyl)tetracycline analogs MIC (µg/mL) Compund ID 8 R A2 SA101 29213 0.125 A5 0.25 A6 0.25 SA161 MRSA,tet (M) 1 4 2 SA158 tet (K) ≤0.0156 0.03125 0.03125 EF159 tet (M) 1 2 2 SP160 tet (M) 0.5 0.25 0.5 MIC (µg/mL) EC107 25922 ≤0.0156 0.125 0.0625 EC155 tet (A) 1 1 2 KP153 tet (A) 0.5 1 1 AB250 0.5 2 2 SM256 7 R SA101 29213 SA161 MRSA,tet (M) SA158 tet (K) EF159 tet (M) SP160 tet (M) EC107 25922 EC155 tet (A) KP153 tet (A) AB250 SM256 A2 Cl 0.125 1 ≤0.0156 1 0.5 ≤0.0156 1 0.5 0.5 0.125 A17 F 0.25 4 ≤0.0156 4 1 0.03125 0.5 0.5 1 0.5 A18 OCH3 0.25 8 0.0625 8 0.5 0.0625 1 1 4 2 A19-A OCF3 2 4 0.5 N/D 4 0.5 2 N/D 2 2 A19-B OCF3 0.125 4 ≤0.0156 N/D ≤0.0156 ≤0.0156 0.25 N/D 0.125 0.125 A20 N(CH3)2 0.5 >32 0.125 32 2 0.125 2 1 16 2 A21-A CF3 0.5 4 0.125 4 2 0.5 4 2 2 1 A21-B CF3 0.125 2 ≤0.0156 2 ≤0.0156 0.03125 0.5 0.25 0.25 0.125 A22 CN 1 32 0.25 N/D 1 0.5 16 N/D 4 16 Compund ID 0.125 1 0.5 Survival (%) MIC (µg/mL) SA161 Contact: Leland Webster Tetraphase Pharmaceuticals, Inc. lwebster@tphase.com EC155 tet (A) 0.5 1 0.5 0.25 KP153 tet (A) 0.5 0.5 0.5 0.25 AB250 SM256 1 0.5 8 4 0.5 0.125 2 1 IV PO 3 mg/kg 30 mg/kg 100% 100% 100% 100% 100% 100% 50% 100% SA: S. aureus; EF: E. faecalis; SP: S. pneumoniae; EC: E. coli; AB: A. baumannii; PA; KP: K. pneumoniae; SM, S. maltophilia. Conclusions: Novel tetracycline analogs with heterocyclic substituents at the C-8 position displayed potent antibacterial activities against a broad range of Gram-positive and Gram-negative pathogens including those with various tetracycline resistance mechanisms. When administered by the oral or intravenous route, the potent in vitro activity translated into promising in vivo efficacy in a murine septicemia model. Selected lead compounds from this novel tetracycline series are undergoing further development. Methods Bacterial Strains. Strains with defined tetracycline resistance mechanisms were obtained from M. Roberts (University of Washington, Seattle, WA). Other strains were from the American Type Culture Collection (ATCC) or Clinical Microbiology Institute (Wilsonville, OR). In vitro Susceptibility. Compounds were dissolved in water and assayed in microtiter plates according to CLSI methodology.1 Mouse Systemic Infection Studies. Mice (n = 6) received treatment via oral gavage (PO) or intravenous (IV) injection 1 hour post intraperitoneal (IP) infection. Percent survival was calculated at termination of study (48 hrs post-dose). Materials. 8-Heterocycle substituted tetracycline analogs were synthesized from appropriately substituted and protected D-ring precursors (such as 6) and the bicyclic enone 72 via a Michael-Dieckmann annulation. A typical synthesis of the 7-chloro-8-(2pyrrolidinyl)tetracycline analogs is shown in Scheme 1. A7 0.5 A8 0.5 A9 0.125 A10 0.125 tigecycline 0.125 1 2 1 0.5 0.25 0.25 2 0.125 0.125 0.0624 1 4 1 0.5 0.0625 4 1 2 2 8 2 4 2 ≤0.0156 0.03125 4 >32 >32 >32 1 4 >32 >32 >32 1 8 N/D >32 2 8 2 N/D Table 4. In vivo antibacterial activity of 7-R-8-(2-pyrrolidinyl)tetracycline analogs Compound ID 2 2 MIC (µg/mL) A2 8 R R R IV 3 mg/kg PO 30 mg/kg 8' A2 Cl methyl 0.125 100% 100% A12 Cl isopropyl 0.25 83% 83% A13 Cl cyclopropyl 0.5 0% 0% A17 F methyl 0.25 100% 100% A23 (CH3 )2N isopropyl 0.25 100% 50% A24 CH3 O isopropyl 0.25 100% 100% 1 Table 2. In vitro antibacterial activity of 7-chloro-8-(2-pyrrolidinyl)tetracycline analogs Compund ID 7 Survival (%) MIC (µg/mL) SA101 29213 SA101 29213 SA161 MRSA,tet (M) SA158 tet (K) EF159 tet (M) SP160 tet (M) EC107 25922 EC155 tet (A) KP153 tet (A) AB250 SM256 0.125 1 ≤0.0156 1 0.5 ≤0.0156 1 0.5 0.5 0.125 Conclusions A range of heterocyclic substituents was incorporated into the tetracycline scaffold at the C8 position using the Tetraphase total synthesis approach. Coupled with substitutions at C7, a series of novel 7,8disubstituted tetracycline analogs was prepared with increased structural diversity and the potential to overcome tetracycline resistance. Among the C8 heterocyclic substituents investigated, the pyrrolidine ring was found to be preferred for optimal antibacterial activity. A number of the new analogs, especially A2, A17, and A24, are highly potent against a broad range of Gram-positive and Gram-negative pathogens in vitro. These compounds have also displayed promising in vivo activity when dosed IV and PO. Several lead compounds from this novel chemical series have demonstrated promising oral bioavailability in PK studies and have been advanced into further preclinical and clinical development. Scheme 1. Synthesis of 7-chloro-8-(2-pyrrolidinyl)tetracycline analogs A11 0.5 2 0.25 0.5 8 4 32 >32 32 4 A12 0.25 1 ≤0.0156 2 0.0625 0.0625 1 1 4 0.5 A13 0.5 1 0.25 2 1 1 4 8 8 4 A14 1 2 0.5 2 2 4 >32 >32 >32 8 A15 0.25 2 0.0313 2 0.25 0.0625 1 1 2 0.5 A16 1 4 0.125 4 1 0.25 2 2 4 2 References & Notes 1) Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard— Ninth Edition. CLSI document M07-A9. Clinical and Laboratory Standards Institute, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898 USA, 2012 2) M.G. Charest, C.D. Lerner, J.D. Brubaker, D.R. Siegel, A.G. Myers, Science, 308, 395 (2005). Printed by 3) We thank Dr. C-H Chen and his colleagues at WuXi Apptec for medicinal chemistry support to this program.