massively parallel sequencing of mate rnal plasma dna in 113 cases
advertisement
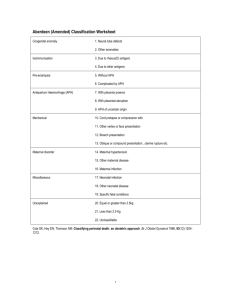
Scuola di specializzazione in Genetica Medica Journal Club 14 gennaio 2014 MASSIVELY PARALLEL SEQUENCING OF MATE RNAL PLASMA DNA IN 113 CASES OF FETAL NUCHAL CYSTIC HYGROMA Bianchi, Diana W. MD; Prosen, Tracy MD; Platt, Lawrence D. MD; Goldberg, James D. MD; Abuhamad, Alfred Z. MD; Rava, Richard P. PhD; Sehnert, Amy J. MD Dr. Fabio Sirchia IGROMA CISTICO ¾ Raccolta di liquido nel tessuto sottocutaneo localizzata in sede retronucale, che può essere secondaria ad una malformazione a carico del sistema linfatico. ¾ Può essere visualizzata ecograficamente come un’area anecogena. IGROMA CISTICO ¾ ¾ ¾ ¾ ¾ Prevalenza: 1/385 Correlata con un aumentato rischio di anomalie cromosomiche, aborti e malformazioni Le sindromi da anomalie cromosomiche sono la causa più frequente. In alcune famiglie l’igroma cistico si presenta come malattia genetica con modalità di trasmissione autosomica recessiva Visibile alla misura della traslucenza nucale (screening ecografico del primo trimestre), in alcuni casi diagnosticato al secondo trimestre di gravidanza. In alcuni casi si risolve spontaneamente prima della nascita ANOMALIE CROMOSOMICHE TRISOMIA 21 MONOSOMIA X TRISOMIA 18 ANEUPLOIDIE E ALTRE ANOMALIE CROMOSOMICHE TRISOMIA 13 DIAGNOSI PRENATALE INVASIVA VILLOCENTESI AMNIOCENTESI NON INVASIVE PRENATAL TESTING (NIPT) In the setting of cystic hygroma could potentially provide fetal karyotype information and thus help in pregnancy management Tested using plasma samples and clinical data from the MatErnal BLood IS Source to Accurately diagnose fetal aneuploidy (MELISSA) study. Clinical Trial in 60 U.S. medical centers under Eligibility criteria for the study included women at approval by local institutional review boards. high risk for fetal aneuploidy undergoing invasive prenatal procedures to determine fetal karyotype. from June 2010 through August 2011 MELISSA Pregnant women 18 years and older between 8 and 22 weeks of gestation undergoing an invasive prenatal procedure to determine fetal karyotype were recruited to participate. who met at least one of the following additional criteria: ¾ age 38 years or older, ¾ positive screening test result for fetal aneuploidy (by serum analytes, nuchal translucency measurement, or both), ¾ presence of ultrasound markers associated with an increased risk for fetal aneuploidy, ¾ a prior aneuploid fetus. ¾ The electronic clinical database of the MELISSA study was searched to identify archived plasma samples from pregnant women carrying fetuses with sonographically diagnosed cystic hygroma at the participating study sites. ¾ All patients with an eligible blood sample that had a singleton pregnancy and a karyotype result, and with selection of the field “cystic hygroma” on the fetal ultrasound page. 74 patients sequenced during the MELISSA trial 113 patients All 113 newly resequenced at the Verinata Health research laboratory using the most recent 39 not previously sequenced version of the sequencing chemistry (Illumina TruSeq 3.0) Patient demographics ¾ 43 of the 113 patients in showed other fetal abnormalities by sonography General Population VS High‐Risk Population Increased overall prevalence of chromosomal abnormalities in the current study: ‐ higher risk profile of patients enrolled in ‐ incomplete karyotype and obstetric outcome data in the other studies MELISSA “2” ILLUMINA TRUSEQ 3.0 Human hg18 Normalized Chromosome value 4 INDEPENDENT CATEGORIES of aneuploidy status Chromosomes 21, 18, and 13 aneuploidy suspected aneuploidy detected normalized chromosome value of greater than 4.0 Monosomy X detected not detected no aneuploidy detected normalized chromosome value of less than 3.0 normalized chromosome value X less than ‐3.0 and an normalized chromosome value Y less than 3.0 Kariotypes results ¾ Sixty‐nine of the 113 (61%) patients had fetuses with abnormal karyotypes, including 30 cases of trisomy 21, 21 cases of monosomy X, 10 cases of trisomy 18 (one was mosaic), 4 cases of trisomy 13, 4 others . ¾ inversion of chromosome 9, inv(9) (p12q13), is a common chromosome variant generally considered to be benign At amniocentesis 46, XX For trisomy 13, the sensitivity was 75% with two of four in the “aneuploidy detected” zone and one of four in the “aneuploidy suspected” zone. specificity was 100% For monosomy X the two with complex mosaicism showed were detected. 20 of 21 samples in the “detected” range for monosomy X. sensitivity: 95.2% specificity: 100%.The case that was not detected had 45,X Trisomy 21 sensitivity 100% with 29 of 30 samples in the “aneuploidy detected” zone and one of 30 in the “aneuploidy suspected” zone. Chromosome 21 specificity was 98.8% with one false‐positive in the “aneuploidy suspected” zone. Ten of 10 fetuses with trisomy 18 were correctly detected for chromosome 18; sensitivity was 100% and trisomy 18 specificity was also 100% Monosomy X 45,X[9]/47,XXX[11]. 18/19 full monosomy X identified 2 mosaic cases identified (45,X,inv(9)(p12q13)[3]/46,XX,inv(9)(p12q13)[7]/46,XX[30]) Massively parallel sequencing agreed with one of the cell lines detected at CVS, but not the amniocentesis or umbilical cord blood results. ¾ Maternal plasma DNA sequencing may reflect aneuploidy present in the fetus, placenta, or the pregnant woman herself. Conclusion ¾ Immediate accessibility by peripheral blood draw, in patients who are risk‐adverse and do not want any invasive testing. For cases in which pregnancy termination is not being considered by the patient (expectant management). ¾ Accurate way of detecting the most prevalent fetal aneuploidies associated with cystic hygroma, high sensitivity and specificity. ¾ Should there be a demise, there would be at least some genetic information available that may help with counseling regarding etiology and recurrence risk. ¾Like with any cell‐free DNA test for fetal aneuploidy, confirmation through an invasive procedure is recommended for such results