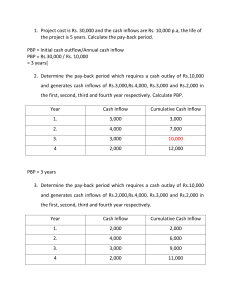
beta-lactamases
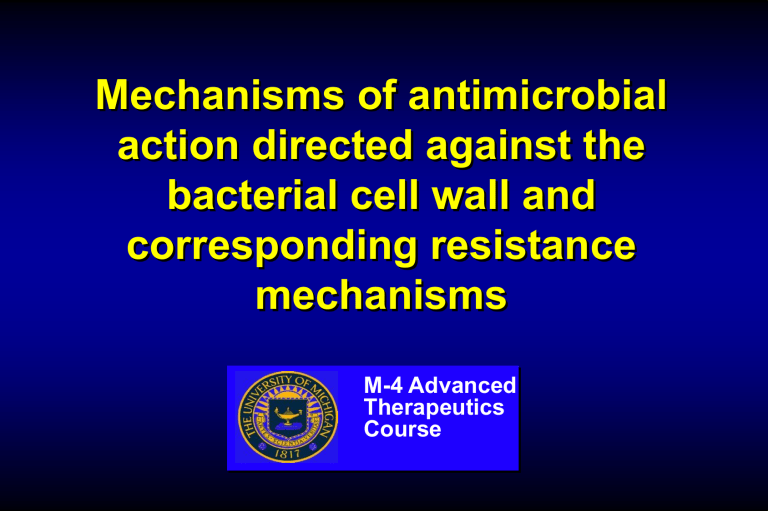
Mechanisms of antimicrobial action directed against the bacterial cell wall and corresponding resistance mechanisms
M-4 Advanced
Therapeutics
Course
Mechanisms of antimicrobial resistance
Drug-modifying enzymes
(e.g.,
- lactamases, aminoglycosidemodifying enzymes)
Altered drug targets
(e.g., PBPs ribosomes,
DNA gyrase)
Altered uptake or accumulation of drug
(e.g., altered porins, membrane efflux pumps)
Subunits for cell wall construction
N-acetylmuramic acid N-acetylglucosamine pentapeptide
D-ala-D-ala
Cell Wall Assembly Second layer of cell wall cross-linked to the lower layer
Layer of cell wall with cross links of 5 glycines
(gray)
A subunit is added to the growing chain
Transpeptidase (PBP) forms a 5-glycine bridge between peptides
Transpeptidase, or PBP (orange sunburst) is bound by beta-lactam antibiotic (light blue) and its activity is inhibited (turns gray)
5-glycine crosslinking bridges cannot form in the presence of a beta-lactam, and the cell wall is deformed and weakened
Mechanisms of beta-lactam resistance
•
Drug-modifying enzymes (beta-lactamases)
–
Gram-positives(e.g., S. aureus) excrete the enzyme
–
Gram-negative (e.g., E. coli) retain the enzyme in the periplasm
•
Overexpression of cell wall synthetic enzymes
– e.g., vancomycin-intermediate S. aureus (VISA)
•
Alteration of the PBPs so antibiotic cannot bind
– e.g., S. pneumoniae, gonococcus
•
Exclusion from the site of cell wall synthesis
–
Porin mutations in the outer membrane of Gramnegative bacteria only (e.g., Ps. aeruginosa)
Beta-lactamases
Beta-lactamases (dark orange) bind to the antibiotics (light blue) and cleave the beta-lactam ring.
The antibiotic is no longer able to inhibit the function of PBP (orange sunburst)
Beta-lactamase activity
Altered drug targets
Vancomycin-intermediate S. aureus
Production of excessive cell wall; the antibiotic cannot keep up
MRSA vancomycin MIC = 2 µg/ml
VISA vancomycin MIC =8 µg/ml
MRSA VISA
Mechanism of vancomycin action
V
D-ala-D-ala
Mechanism of vancomycin resistance
Vancomycin is unable to bind to the D-ala-Dlactate structure
V
D-alaD-lactate
·
June 2002: isolated from the catheter exit site in a chronic dialysis patient
·
The patient had received multiple courses of abx since
April 2001; toe amputation in April 2002 --> MRSA bacteremia
·
VRSA also found at amputation stump wound (with VRE and Klebsiella); not in the patient’s nose
·
Vancomycin MIC >128mcg/ml!! (contains vanA)
·
Sensitive to trim/sulfa, chloro, tetracyclines, Synercid, linezolid
MRSA and penicillin-resistant S. pneumoniae
•
These bacteria are both resistant because they have altered bacterial targets -- penicillin-binding proteins (PBPs or transpeptidases)
•
In MRSA, the altered PBP2 (mecA) gene is acquired by gene transfer from another bacterium.
•
In pneumococci, the alteration in PBP is generated by uptake of DNA released by dead oral streptococci and recombination at the pneumococcal pbp gene to create a new, chimeric protein that does not bind penicillin.
– depicted on the next slide . . .
DNA
Alpha-strep
S. pneumoniae alpha-strep transformation pbp alpha-strep pbp
S. pneumoniae chromosomal pbp; penicillin-sensitive
Chimeric pbp (resistant to penicillin)
Outer membrane permeability in
Gram-negative bacteria
Beta-lactam (blue) enters through an outer membrane porin channel
Altered porin channel prevents access of the antibiotic to the cell wall
Outer membrane
Cell wall
(peptidoglycan)
Inner membrane
Cytoplasm
Bacterium