Pengantar Biokimia Pertemuan 1
advertisement

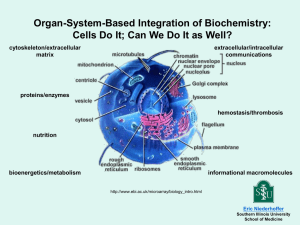
SEJARAH DAN PERKEMBANGAN BIOKIMIA HENDRA WIJAYA WHAT IS BIOCHEMISTRY? • Definition: – Webster’s dictionary: Bios = Greek, meaning “life” “The chemistry of living organisms; the chemistry of the processes incidental to, and characteristic of life.” – WebNet dictionary: “Biochemistry is the organic chemistry of compounds and processes occuring in organisms; the effort to understand biology within the context of chemistry.“ PENGERTIAN BIOKIMIA BIOKIMIA : ilmu yang berhubungan dengan berbagai molekul di dalam sel atau organisme hidup sekaligus dengan reaksi kimianya. BIOS CHEMIOS HIDUP/HAYATI BIOLOGI KIMIA/SENYAWA ORGANIK KIMIA ORGANIK ILMU KIMIA HAYATI (BIOKIMIA) TUJUAN DAN MANFAAT ILMU BIOKIMIA TUJUAN : • Menguraikan semua proses kimiawi pada sel hidup MANFAAT : • Kesejahteraan manusia dan pengembangan ilmu pengetahuan • Dapat dikatakan hampir semua ilmu kehidupan berhubungan dengan Biokimia. BIOCHEMISTRY CAN BE SUBDIVIDED THREE PRINCIPAL AREAS 1. Structural chemistry 2. Metabolism 3. The chemistry of processes and substances that store and transmit biological information (molecular genetics) ISSUES ADDRESSED BY BIOCHEMISTRY 1. What are the chemical and three-deminsional structure of biomolecules? 2. How do biomolecules interact with each other? 3. How does the cell synthesize and degrade biomolecules? 4. How is energy conserved and used by the cell? 5. What are the mechanisms for organizing biomolecules and coordinating their activities? 6. How is genetic information stored, transmitted, and expressed? HISTORY OF BIOCHEMISTRY 1. First to reveal the chemical composition of living organisms. 2. Then to identify the types of molecules found in living organisms. 3. Then to understand how the biomolecules make life to be life. 4. Recent work of biochemist : Biopolymer Structure & Function, Metabolism, Genetic Information SEJARAH BIOKIMIA Pertama, identifikasi unsur kimia penyusun mahluk hidup. Unsur kimia utama penyusun mahluk hidup adalah bahan minor penyusun kerak bumi (kandungan utama 47% O, 28% Si, 7.9% Al, 4.5% Fe, dan 3.5% Ca). Enam unsur utama penyusun sel hidup adalah: C, H, N, O, P, dan S. 99% penyusun sel adalah H, O, N, dan C Unsur Elektron bebas Jumlah Fraksi H 1 2/3 O 2 1/4 N 3 1/70 SEJARAH BIOKIMIA • Kemudian, identifikasi tipe molekul yang ditemukan dalam mahluk hidup • Asam Amino • Nukleotida • Karbohidrat • Lipida Sebagian besar unsur penyusun bahan hidup memiliki berat atom yang rendah; H, O, N dan C adalah unsur dengan berat atom relatif paling kecil yang masing-masing mampu membentuk ikatan tunggal, rangkap, rangkap 3 dan rangkap 4. Unsur dengan berat atom paling ringan membentuk ikatan paling kuat SEJARAH BIOKIMIA Selanjutnya memahami mekanisme biomolekul membuat mahluk hidup menjadi hidup Year 1944 Proteins were thought to carry genetic information 1897 Miescher discovered DNA 1828 Interweaving of the historical traditions of biochemistry, cell biology, and genetics. RELATIONSHIP BETWEEN BIOCHEMISTRY AND OTHER SUBJECTS 1. Organic chemistry, which describes the properties of biomolecules. 2. Biophysics, which applies the techniques of physics to study the structures of biomolecules. 3. Medical research, which increasingly seeks to understand disease states in molecular terms. 4. Nutrition, which has illuminated metabolism by describing the dietary requirements for maintenance of health. RELATIONSHIP BETWEEN BIOCHEMISTRY AND OTHER SUBJECTS 5. Microbiology, which has shown that single-celled organisms and viruses are ideally suited for the elucidation of many metabolic pathways and regulatory mechanisms. 6. Physiology, which investigates life processes at the tissue and organism levels. 7. Cell biology, which describes the biochemical division of labor within a cell. 8. Genetics, which describes mechanisms that give a particular cell or organism its biochemical identity. BIOCHEMISTRY AND LIFE 1. The cell is the fundamental unit of life 2. Prokaryotes and eukaryotes 3. Eukaryotic cells 1. animal cells 2. plant cells (chloroplasts and cell walls) BIOCHEMISTRY AND LIFE • Cells are composed of: – Small molecules – Macromolecules – organelles STRUCTURAL HIERARCHY IN THE MOLECULAR ORGANIZATION OF CELLS BIOCHEMISTRY AND LIFE Percent of Total Cell Weight Number of Types of Each Molecules Water 70 1 Inorganic ions 1 20 Sugars and precursors 3 200 Amino acids and precursors 0.4 100 Nucleotides and precursors 0.4 200 Lipids and precursors 2 50 Other small molecules 0.2 ~200 Macromolecules 22 ~5000 The Approximate Chemical Composition of Bacterial Cell BIOCHEMISTRY AND LIFE • Expect for water, most of the molecules found in the cell are macromolecules, can be classified into four different categories: – – – – Lipids Carbohydrates Proteins Nucleic acids BIOCHEMISTRY AND LIFE 1. Lipids are primarily hydrocarbon structures 2. Carbohydrates, like lipids, contain a carbon backbone, but they also contain many polar hydroxyl (-OH) groups and therefore very soluble in water. 3. Proteins are the most complex macromolecules in the cell. They are composed of linear polymers called polypeptides, which contain amino acids connected by peptide bonds. LIPID STRUCTURE CARBOHYDRATES STRUCTURE BIOCHEMISTRY AND LIFE • Each amino acid contains a central carbon atom attached to four substituents – – – – A carboxyl group An amino group A hydrogen atom An R group • Nucleic acids are the large macromolecules in the cells. They are very long linear polymers, called polynucleotides, composed of nucleotides. AMINO ACIDS STRUCTURES BIOCHEMISTRY AND LIFE • A nucleotide contains : – A five-carbon sugar molecules – One or more phosphate groups – A nitrogenous base • DNA: A, T, G, C • RNA: A, U, G,C DNA CONTAIN FOUR BASES RNA COVALENT STRUCTURE OF DNA WATSON-CRICK BASE PAIRS WATSON-CRICK BASE PAIRS THE DOUBLE HELIX BIOCHEMICAL ENERGY • All cellular functions re quire energy. • The most-important chemical form of energy in most cells is ATP, adenosine 5’-triphosphate. • ATP ADP + Pi • Most ATP synthesis occurs in chloroplasts and mitochondria ADP AND ATP STRUCTURES ENERGY TRANSFER ENERGY TRANSFER Transfer of Information from DNA to Protein DNA RNA Protein TRANSFER OF INFORMATION FROM DNA TO PROTEIN