a gene
advertisement

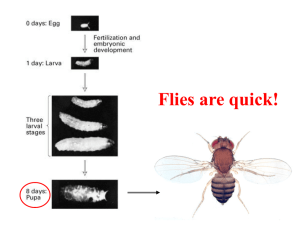
Model organisms: mice • vertebrates! • mice are ~ 3 inches long, can keep many mice in a room. • generation time is ~ 3 months, so genetics can be done • history - scientists have worked with mice for 100 years • genetic tools - can introduce extra genes or remove a specific gene, then study the effect on development • Disadvantages: development inside the mother, hard to see. Expensive! Large Genome = 3 Gb The mouse provides a superb model for human development and disease because we share virtually ALL of our genes and use them in similar ways Kit gene Figure 1.22 Genetic analysis: creating transgenic mice Problem: Find a cell line that can grow in tissue culture but also retains the potential to become part of a real embryo. Solution: Embryonic stem cells blastocyst inner cell mass Embryonic stem cells: blastocyst-stage cells (from inner cell mass) that have been coaxed into growing in culture Blastocyst stage cells can be easily incorporated into a different blastocyst stage embryo, allowing production of chimeric mice mom and dad have white fur mom and dad have black fur mouse with 4 parents!! Fig. 8.26 A mouse with 3 of its parents (6 total!) Fig. 8.26 Adding a gene: Producing Transgenic Mice Production of Transgenic Mice Embryonic stem cells (ES cells) are then incorporated into blastocysts, with the hope that they “go germline”. If so, a line is created Production of Transgenic Mice Production of Transgenic Mice Recipe to "knockout" a gene: A normal cell has two copies of a gene (ie. BMP7) RNA Gene X RNA Gene X 1. Insert gene for resistance to the drug neomycin into the middle of gene X, destroying its function. (Gene X is contained in a DNA plasmid.) 2. Introduce gene X KO plasmid into ES cells and use homologous recombination to replace one of the wildtype copies of gene X with mutant gene. Mario Cappechi Neo resistance gene No RNA Gene X RNA Oliver Smithies Gene X Technique for Gene Targeting #2 #1 #3 Now you have heterozygous ES cells--how do you make a homozygous mutant mouse? #4 #5 Now you have a chimeric mouse… #6 #7 Sometimes the effects are dramatic! Wild-type BMP7 knockout Morphological Analysis of Bmp7 Knockout Mice Sometimes the effects are not dramatic --no phenotype! Mouse models of human disease allow us to design and test new treatments CFTR and cystic fibrosis Oliver Smithies remember me? Wildtype Ultrabithorax mutant The Homeotic genes in Drosophila ANT-C BX-C Fig 6.35 Ed Lewis had predicted that the homeotic genes would shape the body plans of all animals In vertebrates the Hox genes have been duplicated, creating four clusters Figure 8.30 Different Hox genes are expressed at different places along the anterior-posterior body axis Knocking out Hoxc8 Figure 8.30 Partial transformation of the first lumbar vertebra into a thoracic vertebra by knockout of the Hoxc8 gene Genetic analysis of Hox genes is more complicated in mice Knocking out Hoxa10, Hoxc10 & Hoxd10 paralog group Figure 8.30 The duplication of the Hox clusters means that in the mouse, Hox genes work together to give each body region its own identity wildtype Hoxa10 Hoxc10 Hoxd10 triple mutant Figure 8.32 Lumbar vertebrae transformed to thoracic vertebrae Remember the segment-polarity genes wingless and engrailed? Wg En Retroviruses can also cause cancer by inserting next to and thus activating the expression of proto-oncogenes retroviral insertion sites in different tumors Transcribe to mRNA 5 kilobases exons wnt-1 gene Wnt-1, The mouse homolog of wingless, is normally expressed at the midbrain-hindbrain junction Expresses Wnt-1 Expresses En-1 Wildtype brain Structures lost in Wnt-1 mutant Expresses Wnt-1 Expresses En-1 Wildtype brain Brain of Wnt-1 mutant Pax6 Rules of Evidence What type of experiment is this? Pax6 regulates eye development in flies, squid, mice, and us Normal eye iris no iris Aniridia eye small eye mutant mouse When eyeless (Pax6 homolog) is expressed at the ends of fly legs, extra eyes form there! When squid Pax6 homolog is expressed at the ends of fly legs, also see extra eyes! ectopic eye (fly Pax6) ectopic eye (squid Pax6) The Pax-3 gene is altered in a classic mouse mutation Wild-type Splotch mutant Mutations in Pax3 lead to Waardenburg Syndrome I. • • • • dominant mutation eyes can be different colors white patch of hair (forelock) deafness Why Models Matter The Example of Mutation of the Kit gene in humans and mice “Piebaldism” • Affected individuals are anemic, sterile, deaf, and lack pigment in certain skin cells • Kit encodes a receptor tyrosine kinase and is required for cell proliferation in neural crest, blood, and germ cells Figure 1.22 White spotting and Steel: Connecting classic mouse mutations to stem cells and cancer An example of stem cells: the blood cell lineage Cells lacking signal behave differently than cells lacking receptor Mosaics can + + help us + understand + gene and thus protein function + + + + + + Thanks, I needed that! + + + + + + mutant + mutant + + mutant + + + + + + + + + + + If mutant cells lack signal, they can be rescued by wildtype neighbors which make signal. What? I can't hear you! + + + + + + mutant + mutant + + mutant + + + + + + + + + If mutant cells lack receptor, they cannot be rescued by wildtype neighbors which make signal. White-spotting and Steel: Which is signal and which is receptor?? Experiment #1 Put blood cells from Steel homozygous mutant embryos into a wild-type host. Experiment #2 Put blood cells from White-spotting homozygous mutant embryos into a wildtype host. The mutant blood cells migrated to the bone marrow. These mutant blood cells did not migrate to the bone marrow. Steel is the Signal- Mutant cells can still receive information White-spotting is the receptor- Mutant cells cannot receive information Steel encodes a diffusible ligand and White-spotting (Kit) encodes its transmembrane receptor tyrosine kinase domain activated when Steel binds, phosphorylating target proteins PAX3 activates Mitf melanin genes Fig. 3.22 Mutations in MITF lead to Waardenburg Syndrome II.