post-transcription
advertisement

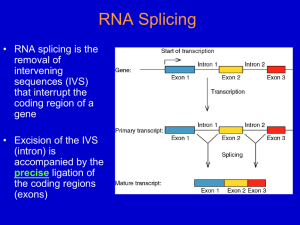
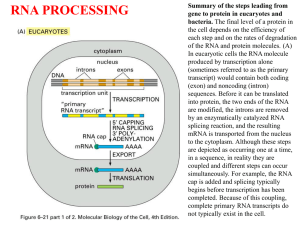
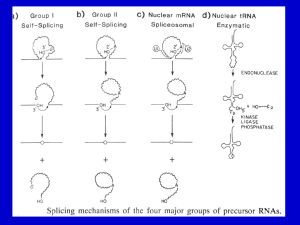
Transcription and Post-transcription Modification 李 希 分子医学教育部重点实验室 lixi@shmu.edu.cn Post-transcriptional Processing of RNA Making ends of RNA RNA splicing Primary Transcript • Primary Transcript-the initial molecule of RNA produced--- hnRNA (heterogenous nuclear RNA ) • In prokaryotes, DNA →RNA →protein in cytoplasm concurrently • In eukaryotes nuclear RNA >> Cp RNA Processing of eukaryotic pre-mRNA For primary transcripts containing multiple exons and introns, splicing occurs before transcription of the gene is complete--cotranscriptional splicing. Human dystrophin gene has 79 exons, spans over 2,300-Kb and requires over 16 hours to be transcribed! Types of RNA processing A) Cutting and trimming to generate ends: rRNA, tRNA and mRNA B) Covalent modification: Add a cap and a polyA tail to mRNA Add a methyl group to 2’-OH of ribose in mRNA and rRNA Extensive changes of bases in tRNA C) Splicing pre-rRNA, pre-mRNA, pre-tRNA by different mechanisms. The RNA Pol II CTD is required for the coupling of transcription with mRNA capping, polyadenylation and splicing 1. The coupling allows the processing factors to present at high local concentrations when splice sites and poly(A) signals are transcribed by Pol II, enhancing the rate and specificity of RNA processing. 2. The association of splicing factors with phosphorylated CTD also stimulates Pol II elongation. Thus, a pre-mRNA is not synthesized unless the machinery for processing it is properly positioned. Time course of RNA processing 5’ and 3’ ends of eukaryotic mRNA 5’-UTR Add a GMP Methylate it and 1st few nucleotides 3’-UTR Cut the pre-mRNA and add A’s Capping of pre-mRNAs • Cap=modified guanine nucleotide • Capping= first mRNA processing event - occurs during transcription • CTD recruits capping enzyme as soon as it is phosphorylated • Pre-mRNA modified with 7-methyl-guanosine triphosphate (cap) when RNA is only 25-30 bp long • Cap structure is recognized by CBC(cap-binding complex) • • • stablize the transcript prevent degradation by exonucleases stimulate splicing and processing Capping of the 5’ end of nascent RNA transcripts with m7G • The Existing in a single complex Sometimes methylated Sometimes methylated cap is added after the nascent RNA molecules produced by RNA polymerase II reach a length of 2530 nucleotides. Guanylyltransferase is recruited and activated through binding to the Ser5-phosphorylated Pol II CTD. • The methyl groups are derived from Sadenosylmethionine. • Capping helps stabilize mRNA and enhances translation, splicing and export into the cytoplasm. Consensus sequence for 3’ process AAUAAA: CstF (cleavage stimulation factor F) GU-rich sequence: CPSF (cleavage and polyadenylation specificity factor) Polyadenylation of mRNA at the 3’ end CPSF: cleavage and polyadenylation specificity factor. CStF: cleavage stimulatory factor. CFI & CFII: cleavage factor I & II. PAP: poly(A) polymerase. PABPII: poly(A)-binding protein II. RNA is cleaved 10~35-nt 3’ to A2UA3. The binding of PAP prior to cleavage ensures that the free 3’ end generated is rapidly polyadenylated. PAP adds the first 12A residues to 3’-OH slowly. Binding of PABPII to the initial short poly(A) tail accelerates polyadenylation by PAP. Poly(A) tail stabilizes mRNA and enhances translation and export into the cytoplasm. The polyadenylation complex is associated with the CTD of Pol II following initiation. Functions of 5’ cap and 3’ polyA • Need 5’ cap for efficient translation: – Eukaryotic translation initiation factor 4 (eIF4) recognizes and binds to the cap as part of initiation. • Both cap and polyA contribute to stability of mRNA: – Most mRNAs without a cap or polyA are degraded rapidly. – Shortening of the polyA tail and decapping are part of one pathway for RNA degradation in yeast. mRNA Half-life • t½ seconds if seldom needed • t½ several cell generations (i.e. ~48-72 h) for houskeeping gene • ≈avg 3 h in eukaryotes • ≈avg 1.5 min in bacteria Poly(A)+ RNA can be separated from other RNAs by fractionation on Sepharose-oligo(dT). Split gene and mRNA splicing The discovery of split genes (1977) 1993 Noble Prize in Medicine To Dr. Richard Robert and Dr. Phillip Sharp Background: Adenovirus has a DNA genome and makes many mRNAs. Can we determine which part of the genome encodes for each mRNA by making a DNA:RNA hybrid? Experiment: Isolate Adenovirus genomic DNA, isolate one adenovirus mRNA, hybridize and then look by EM at where the RNA hybridizes (binds) to the genomic DNA. Surprise: The RNA is generated from 4 different regions of the DNA! How can we explain this? Splicing!! mRNA DNA The matured mRNAs are much shorter than the DNA templates. Exon and Intron • Exon is any segment of an interrupted gene that is represented in the mature RNA product. • Intron is a segment of DNA that is transcribed, but removed from within the transcript by splicing together the sequences (exons) on either side of it. Exons are similar in size Introns are highly variable in size GT-AG rule • GT-AG rule describes the presence of these constant dinucleotides at the first two and last two positions of introns of nuclear genes. • Splice sites are the sequences immediately surrounding the exon-intron boundaries • Splicing junctions are recognized only in the correct pairwise combinations The sequence of steps in the production of mature eukaryotic mRNA as shown for the chicken ovalbumin gene. The consensus sequence at the exon–intron junctions of vertebrate pre-mRNAs. 4 major types of introns 4 classes of introns can be distinguished on the basis of their mechanism of splicing and/or characterisitic sequences: – Group I introns in fungal mitochondria, plastids, and in pre-rRNA in Tetrahymena (self-splicing) – Group II introns in fungal mitochondria and plastids (self-splicing) – Introns in pre-mRNA (spliceosome mediated) – Introns in pre-tRNA Group I and II introns The sequence of transesterification reactions that splice together the exons of eukaryotic pre-mRNAs. Splicing of Group I and II introns • Introns in fungal mitochondria, plastids, Tetrahymena prerRNA • Group I – Self-splicing – Initiate splicing with a G nucleotide – Uses a phosphoester transfer mechanism – Does not require ATP hydrolysis. • Group II – self-splicing – Initiate splicing with an internal A – Uses a phosphoester transfer mechanism – Does not require ATP hydrolysis Self-splicing in pre-rRNA in Tetrahymena : T. Cech et al. 1981 + Exon 1 Intron 1 Exon 2 Exon 1 Exon 2 Intron 1 •Products of splicing were resolved by gel electrophoresis: + + + pre-rRNA + Nuclear extract Additional proteins + + GTP + + are NOT needed for pre-rRNA Spliced exon Intron circle Intron linear splicing of this prerRNA! Do need a G nucleotide (GMP, GDP, GTP or Guanosine). The sequence of reactions in the self-splicing of Tetrahymena group I intron. Where is the catalytic activity in RNase P? RNase P is composed of a 375 nucleotide RNA and a 20 kDa protein. The protein component will NOT catalyze cleavage on its own. The RNA WILL catalyze cleavage by itself !!!! The protein component aids in the reaction but is not required for catalysis. Thus RNA can be an enzyme. Enzymes composed of RNA are called ribozymes. Hammerhead ribozymes • A 58 nt structure is used in self-cleavage • The sequence CUGA adjacent to stemloops is sufficient for cleavage 5' 3' AA A GGCC CCGG A CG U A C G AUC U G GU A Bond that is cle ave d. ACCAC C UGGUG CUGA is r e quir e d for catalys is Mechanism of hammerhead ribozyme • The folded RNA forms an active site for binding a metal hydroxide • Attracts a proton from the 2’ OH of the nucleotide at the cleavage site. • This is now a nucleophile for attack on the 3’ phosphate and cleavage of the phosphodiester bond. 1989 Nobel Prize in chemistry, Sidney Altman, and Thomas Cech Distribution of Group I introns • Prokaryotes – eubacteria (tRNA & rRNA), phage • Eukaryotes – lower (algae, protists, & fungi) • nuclear rRNA genes, organellar genes, Chlorella viruses – higher plants: organellar genes – lower animals (Anthozoans): mitochondrial • >1800 known, classified into ~12 subgroups, based on secondary structure Splicing of pre-mRNA • The introns begin and end with almost invariant sequences: 5’ GU…AG 3’ • Use ATP hydrolysis to assemble a large spliceosome (45S particle, 5 snRNAs and 65 proteins, same size and complexity as ribosome) • Mechanism is similar to that of the Group II fungal introns: – Initiate splicing with an internal A – Uses a phosphoester transfer mechanism for splicing Initiation of phosphoester transfers in pre-mRNA • Uses 2’ OH of an A internal to the intron • Forms a branch point by attacking the 5’ phosphate on the first nucleotide of the intron • Forms a lariat structure in the intron • Exons are joined and intron is excised as a lariat • A debranching enzyme cleaves the lariat at the branch to generate a linear intron • Linear intron is degraded Involvement of snRNAs and snRNPs • snRNAs = small nuclear RNAs • snRNPs = small nuclear ribonucleoproteins particles (snRNA complex with protein) • Addition of these antibodies to an in vitro premRNA splicing reaction blocked splicing. • Thus the snRNPs were implicated in splicing Role of snRNPs in RNA splicing • Recognizing the 5’ splice site and the branch site. • Bringing those sites together. • Catalyzing (or helping to catalyze) the RNA cleavage. RNA-RNA, RNA-protein and protein-protein interactions are all important during splicing snRNPs U1, U2, U4/U6, and U5 snRNPs – Have snRNA in each: U1, U2, U4/U6, U5 – Conserved from yeast to human – Assemble into spliceosome – Catalyze splicing Splicing of pre-mRNA occurs in a “spliceosome” an RNA-protein complex spliceosome (~100 proteins + 5 small RNAs) pre-mRNA spliced mRNA The spliceosome is a large protein-RNA complex in which splicing of pre-mRNAs occurs. Assembly of spliceosome • snRNPs are assembled progressively into the spliceosome. – U1 snRNP binds (and base pairs) to the 5’ splice site – BBP (branch-point binding protein) binds to the branch site – U2 snRNP binds (and base pairs) to the branch point, BBP dissociates – U4U5U6 snRNP binds, and U1 snRNP dissociates – U4 snRNP dissociates • Assembly requires ATP hydrolysis • Assembly is aided by various auxiliary factors and splicing factors. Some RNA-RNA hybrids formed during the splicing reaction Steps of the spliceosomemediated splicing reaction Assembly of spliceosome A schematic diagram of six rearrangements that the spliceosome undergoes in mediating the first transesterification reaction in pre-mRNA splicing. The spliceosome cycle The Significance of Gene Splicing • The introns are rare in prokaryotic structural genes • The introns are uncommon in lower eukaryote (yeast), 239 introns in ~6000 genes, only one intron / polypeptide • The introns are abundant in higher eukaryotes (lacking introns are histons and interferons) • Unexpressed sequences constitute ~80% of a typical vertebrate structural gene Errors produced by mistakes in splice-site selection Mechanisms prevent splicing error • Co-transcriptional loading process • SR proteins recruit spliceosome components to the 5’ and 3’ splice sites • SR protein = Serine Arginine rich protein • ESE = exonic splicing enhancers • SR protein regulates alternative splicing Alternative splicing • Alternative splicing occurs in all metazoa and is especially prevalent in vertebrate Five ways to splice an RNA Regulated alternative splicing Different signals in the pre-mRNA and different proteins cause spliceosomes to form in particular positions to give alternative splicing Alternative splicing can generate mRNAs encoding proteins with different, even opposite functions Fas ligand Fas 5 6 7 (membraneassociated) Fas pre-mRNA 5 (+) 6 7 APOPTOSIS (programmed cell death) (-) Fas ligand 5 7 Soluble Fas (membrane) Drosophila Dscam gene contains thousands of possible splice variants Alternative possibilities for 4 exons leave a total number of possible mRNA variations at 38,016. The protein variants are important for wiring of the nervous system and for immune response. Cis- and Trans-Splicing Cis-: Splicing in single RNA Trans-: Splicing in two different RNAs Y-shaped excised introns (cis-: lariat) Occur in C. elegance and higher eukaryotes but it does in only a few mRNAs and at a very low level Same splicing mechanism is employed in trans-splicing pre-mRNA splicing trans-mRNA splicing spliced leader Spliced leader contains the cap structure! RNA editing • RNA editing is the process of changing the sequence of RNA after transcription. • In some RNAs, as much as 55% of the nucleotide sequence is not encoded in the (primary) gene, but is added after transcription. • Examples: mitochondrial genes in Trypanosomes (锥虫) • Can add, delete or change nucleotides by editing Two mechanisms mediate editing • Guide RNA-directed uridine insertion or deletion • Site-specific deamination Insertion and deletion of nucleotides by editing • Uses a guide RNA (in 20S RNP = editosome) that is encoded elsewhere in the genome • Part of the guide RNA is complementary to the mRNA in vicinity of editing Trypanosomal RNA editing pathways. (a) Insertion. (b) Deletion. Mammalian example of editing The C is converted to U in intestine by a specific deaminating enzyme, not by a guide RNA. Cutting and Trimming RNA • Can use endonucleases to cut at specific sites within a longer precursor RNA • Can use exonucleases to trim back from the new ends to make the mature product • This general process is seen in prokaryotes and eukaryotes for all types of RNA The posttranscriptional processing of E. coli rRNA. RNase III cuts in stems of stem-loops 16S rRNA 23S rRNA RNase III No apparent primary sequence specificity - perhaps RNase III recognizes a particular stem structure. Eukaryotic rRNA Processing • The primary rRNA transcript (~7500nt, 45S RNA) contains 18S, 5.8S and 28S • Methylation occur mostly in rRNA sequence 80%: O2-methylribose, 20%: bases (A or G) • peudouridine 95 U in rRNA in human are converted to Y’s may contribute rRNA tertiary stability Transfer RNA Processing • Cloverleaf structure • CCA: amino acid binding site • Anticodon • ~60 tRNA genes in E. coli A schematic diagram of the tRNA cloverleaf secondary structure. Endo- and exonucleases to generate ends of tRNA • • • Endonuclease RNase P cleaves to generate the 5’ end. Endonuclease RNase F cleaves 3 nucleotides past the mature 3’ end. Exonuclease RNase D trims 3’ to 5’, leaving the mature 3’ end. Splicing of pre-tRNA • Introns in pre-tRNA are very short (about 10-20 nucleotides) • Have no consensus sequences • Are removed by a series of enzymatic steps: – Cleavage by an endonuclease – Phosphodiesterase to open a cyclic intermediate and provide a 3’OH – Activation of one end by a kinase (with ATP hydrolysis) – Ligation of the ends (with ATP hydrolysis) – Phosphatase to remove the extra phosphate on the 2’OH (remaining after phosphodiesterase ) Steps in splicing of pre-tRNA + OH 5’ 1. Endonuclease Intron of 10-20 nucleotides P 2’,3’ cyclic phosphate + Excised intron 2. Phosphodiesterase 3. Kinase (ATP) 4. Ligase (ATP) 5. Phosphatase Spliced tRNA CCA at 3’ end of tRNAs • All tRNAs end in the sequence CCA. • Amino acids are added to the CCA end during “charging” of tRNAs for translation. • For most eukaryotic tRNAs, the CCA is added after transcription, in a reaction catalyzed by tRNA nucleotidyl transferase. All of the four bases in tRNA can be modified Pathologies resulting from aberrant splicing can be grouped in two major categories • Mutations affecting proteins that are involved in splicing Examples: Spinal Muscular Atrophy Retinitis Pigmentosa Myotonic Dystrophy • Mutations affecting a specific messenger RNA and disturbing its normal splicing pattern Examples: β-Thalassemia Duchenne Muscular Dystrophy Cystic Fibrosis Frasier Syndrome Frontotemporal Dementia and Parkinsonism Intron Advantage? • One benefit of genes with introns is a phenomenon called alternative splicing • A pre-mRNA with multiple introns can be spliced in different ways – This will generate mature mRNAs with different combinations of exons • This variation in splicing can occur in different cell types or during different stages of development Intron Advantage? • The biological advantage of alternative splicing is that two (or more) polypeptides can be derived from a single gene • This allows an organism to carry fewer genes in its genome Do you believe? RNA Interference and Interference RNA RNA interference or RNAi is a remarkable process whereby small noncoding RNA silence specific genes. - RNAi was first observed in plant in immune response to viral pathgens. - MicroRNA regulate gene expression in organisms from nematode to man. Nobel Prize in Physiology or Medicine 2006, Andrew . Fire and Craig . Mello RNA Interference: A Mechanism for Silencing Gene Expression 1. Small dsRNA fragments can silence the expression of a matching gene. This is RNA interference (RNAi), recently discovered in C. elegans. a. Injecting dsRNA into adult worms results in specific loss of the corresponding mRNA in the worm and its progeny. b. RNAi also occurs in many other organisms, where it protects against viral infection and regulates developmental processes. 2. RNAi is highly specific and sensitive, with only a few molecules of dsRNA needed, making it an excellent research tool. Comparison of siRNA and miRNA siRNA miRNA Precursor Endogenous or exogenous dsRNA Endogenous transcript Structure dsRNA ssRNA Function mRNA cleavage Translation inhibition and mRNA cleavage Target mRNA perfect complimentarity Imperfect complimentarity development Inhibit transpon and Biological virus infection Foreign DNA and Transgene Foreign DNA and Transgene Aberrant sense RNA RdRP dsRNA Dicer siRNAs Heterochromatin formation and Transcriptional silencing Nature 2004, Vol 431, Sept.16:343 Self splicing miRNA Mitrons :Short intronic hairpins RNA Ploymerase II or III pri-miRNA No need of Drosha Splicing machinery Lariat debranching enzyme pre-miRNAs Cell 130, July 13 2007: 89-100 Dicer Micro RNAs (MiRNAs) ~22NTs RISC lncRNA functions Something I may not care , but you have to.