Apoptosis - Rci.rutgers.edu

Lecture 19
Homework Review
Apoptosis and Cancer
Office Hours This Week: Today ~ 5:30- 7:30pm
Next Two Lectures:
Cell-Cell Interactions/Tissues
Early Development and Stem Cells
For Exam III- You are not responsible for any material in assigned chapters relating to Plants!
Apoptosis:
Regulated Cell Death
Role in Killing of Unneeded, Damaged, or Potentially Deleterious
Cells
Occurs in Embryonic and Adult Tissues
Proteins Involved are Always Present in Cells- Needs to Be
Activated by Stimuli
Can Result From:
Developmental Cues
Withdrawl of Essential Growth Factors
DNA Damage
Various Cell Stresses
Programmed Cell Death
• Cell Death Occurring at a Defined Point in
Development
• Usually proceeds by Apoptosis
Mouse Paws
Not All Cell Death is Apoptotic
Oncosis and Necrosis:
Unregulated Cell Death Due to Injury
Cell Swells (Oncosis)
Nucleus Swells
Disruption of Organelles and
Rupture/Release of Contents
Contents Released into
Extracellular Space
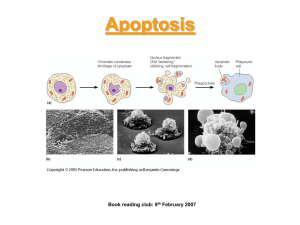
Apoptosis:
An Active Regulated Process
DNA Fragmentation
Chromatin Condensation
Fragmentation of Nucleus
Cell Shrinks
Formation of Membrane Enclosed
Fragments called Apoptotic Bodies
Recognition and Engulfment by Phagocytic Cells or Neighboring Cells
The Morphological Changes of Apoptosis Are
Orchestrated by Caspases
Cysteine Proteases that cleave at Aspartic Acid Residues
Activate Apoptosis by Cleaving Specific Substrates
Present but inactive in cells
Two Main Types of Caspases:
1) Initiators-
Need to dimerize to become active “induced proximity”
2) Executioner (Effector) - Need to be proteolytically cleaved to become active
- Cleavage is usually Mediated by Initiator Caspases
Zymogens
Caspase Activation Amplification Cascade
Once Executioners are Activated their
Key Targets of Proteolysis Include:
1)An Inhibitor of a DNAse-
Leads to Fragmentation of DNA
2)Nuclear Lamins-
Leads to Fragmentation of Nucleus
3)Other Cytoskeletal Associated Proteins-
Leads to Disruption of Cytoskeleton and
Cell Fragmentation
4)Additional Caspases
Main Pathways Regulating Caspase
Activation During Apoptosis
Intrinsic Pathway- Mitochondrial Mediated
Major Pathway in Mammalian Cells
– Outer Mitochondrial Membrane Permeabilization (MOMP)
– Release of Cytochrome C from Mitochondrial Intermembrane
Space into Cytosol
– Apoptosome Formation- Activation of Initiator Caspase
– Effector Caspases Activated
Extrinsic Pathway- Signaling through Death Receptors
– Ligand Bound Death Receptors
– Adaptor Protein Association
– Initiator Caspase Recruitment and Activation
– Effector Caspases Activated
Intrinsic Pathway of Apoptosis Activation
MOMPs cytochrome c Release
Apoptosome Formation:
Adaptor (Apaf1), dATP cytochrome c and procaspase complex
Association of Adaptor with Procaspase allows
Procaspase self cleavage
Active Initiator Caspase
Cleaves Effector
Caspases
Which now Cleave
Targets
Critical Regulators of Cell Death
Bcl-2 Family –
Regulate whether MOMPs Occurs
Anti-Apoptotic Factors - Death Inhibitors
A) Function to Inhibit MOMPs by Pro Apoptotic Factors
Pro-Apoptotic Factors- Death Activators
A) Bind and inhibit Death Inhibitors
B) Directly cause Permeabilization of MOM to
Stimulate Release of Cytochrome C ( BAX AND BAK)
IAP Family (Inhibitor of Apoptosis)
Bind Procaspases prevent activation
Bind Caspases and inhibit Activity
Survival Factor Signaling is
Required to Prevent Apoptosis
Programmed Cell Death in Neuronal Development
Survival Factors Signaling Can Function to
Keep Anti-Apoptotic Factor Bcl-2 Active
No Survival Signal
Bcl-2 Complexes with
Bad
Can’t prevent
BAK and BAX
Mediated
MOMPs
Extrinsic Pathway of Apoptosis Activation:
Signaling through the Death Receptors
Ligand Bound Death Receptors
Adaptor Protein and
Procaspase Recruitment
Initiator Caspase Activation
Effector Caspases Activated
Target cells :
Viral Infected Cells or
Cancer Cells
Removal of Excess
Lymphocytes after
Infection
Cancer
Cancer is a Disease of Cells that Proliferate at Inappropriate Times and
Locations in the Body.
Tumors (Neoplasms) - Masses of cells derived from a single abnormally proliferating cell. Tumors are Clonal
1. Benign- Noninvasive, Do not affect other tissues
2. Malignant- Cancerous, Locally Invasive and May Spread
Tumors are classified by cell type from which they arise.
1. Carcinoma90% of human cancersMalignacy of Epithelial Cells
2. Sarcomas
–
Rare, Solid tumors of connective tissue, such as bone, muscle, cartilage, and fibrous tissue.
3. Leukemias and Lymphomas7% of cancers, Blood forming cells and cells of immune system
4. NeuroectodermalCells of central or peripheral nervous system
The Development of Cancer is a Multistep Process
Initial Cell
Proliferating
Abnormally
Typically requires four to six different mutations
Tumorigenesis
Occurs by Clonal Expansion:
Yields Population of Cells
More Abnormal and
More Adapted
Proliferate, Survive,
Invade and Metastasize
Intravasation:
Malignant cells gain access to blood vessels and lymphatic system and spread
Metastasis:
Malignant cells
Establish in distant organs
Cancer Cells are Characterized by Several Distinct Properties when Grown in vitro
Key Characteristic
Contact Inhibition of Growth
Growth Factor Requirements
Anchorage Dependence
Cell Cycle Checkpoints
Karyotypic Profile
Proliferative Life Span
Normal Cell
Present
High
Cancer Cell
Absent
Low
Present Absent
Intact Absent
Normal Abnormal
Finite Indefinite
Cancer cells are also:
Defective in Differentiation
Fail to Undergo Apoptosis
Cancer Cells Are Created when
Certain Genes are Mutated
Mutations can be Inherited, Introduced by Viruses, or Result of DNA
Damage (exposure to a mutagen)
1. Oncogenes - Gene whose presence can trigger inappropriate cell proliferation.
Example: ras, bcl-2
(Normal version of gene: Proto-oncogene)
2. Tumor SuppressorsGene whose absence or inactivation can lead to cancer
Usually Function to Block Cell Cycle Progression
Example: p53, Rb
DNA Repair Genes- Increase Rate of Mutation, provide opportunity for mutation in growth controlling genes, increase rate of tumor progression
Cancer Cells Are Created When
Certain Genes are Mutated
Activation of Oncogene
Can Also Occur
By:
Inhibition of Tumor Suppressor Genes
Overexpression of Protooncogene
Translocations that create hybrid proteins
Oncogenes are Found in Mitogen and
Growth Factor Signal Transduction Pathways
Mutation of Proto-oncogene-
Constitutively Active Downstream
Signal Transduction Pathway
Inactivation of Tumor Suppressor Rb
Common Target for Viruses that
Cause Tumors
(along with p53)
Cancer Cells Exhibit Unlimited
Proliferative Ability
Cancer cells avoid senescence by inactivating tumor suppressor genes, p53 and Rb.
Cancer Cells will continue to divide for a period of time
Crisis Point – Large number of Cancer Cells Die- Result of catastrophic rearrangements- due to lack of telomerase
Rare Occasion A Cell Survives- It is Immortalized.
At some point- derepressed telomerase expression
~ 90% of cancer cells express significant levels of telomerase