Chromosomes and Mitosis
Lecture 6
1 Chromosomal Basis of Heredity
• A gene is a unit
of heredity
• Genes are
carried on DNA
• DNA is
contained
within
chromosomes as
chromatin
Chromosomes replicate during cell
division
The chromosome
complement
Chromosome analysis
Cri Du Chat results
from loss of a small
piece of chromosome 5
Gene Map
Chromosome
pairs
Non-identical genes
Sex
chromosomes
• These determine the sex of an
individual
– Two X chromosomes make a female
– One X and one Y a male
Two types of Cell Division
• Cells divide for two reasons
– To create genetically identical
copies of themselves
46
46
• This is
mitosis
–
To create gametes that
contain half of the
chromosomes of the original
cell
• This is meiosis
23
46
46
23
23 23
The Cell
Cycle
S phase
Replication
Condensation
Schematic
DNA replication
Duplex DNA begins Replicating
Replication bubbles merge creating two duplexes
Mitosis
The stages of Mitosis
Prophase Detail
Prometaphase
Metaphase
Anaphase
Telophase
The sum total of
the process
Karyotypes
Chromosome
Length
Chromosome appearance
Meiosis and Gametogenesis
Somatic and Germline cells
• Development of a fertilized egg into an adult results
in two distinct types of cells
– Somatic cells
• These create all tissues and organs of the adult except for cells
destined to become sperm or egg
• They can only undergo mitosis
– Germline cells
• The final differentiated form of these cells are mature gametes:
the sperm and egg
• These cells undergo mitosis until gametogenesis
– They then undergo meiosis
Meiosis
Meiosis is required for gametogenesis
Meiosis I
Somatic cells
Germline Cells
Interphase I and
Prophase I
Leptotene
Prophase I
Zygotene
Prophase I
Pachytene
Prophase I
Diplotene
Recombination
And on the molecular level
Metaphase I
and
anaphase I
Meiosis I is the reduction
division
Non-disjunction
Telophase
I
Cytokinesis
sperm formation
oocyte formation
Meiosis II
A comparison
of meiosis and
mitosis
Mitosis
Maintains
Meiosis
Reduces
1
2
Cells resulting
2
4
Cells involved
Somatic
Germline
Chromosome
number
Nuclear Divisions
Relationship to Gametogenesis
Sperm and Egg
formation
Gametogenesis
• Entry of a single sperm into an egg prevents entry of
other sperm
• The DNA of sperm and egg are initially kept
separate in “pronuclei” of the zygote
• Timing of a pregnancy extends from the “last
menstrual period” (LMP) rather than the time of
fertilization
Fertilization
Mitotic Non-disjunction
Cell cycle and apoptosis
• Cells undergo 3 controlled processes
– The first two are part of the cell cycle, the last an exit from the cell
cycle
– Division (the cell cycle)
– Quiescence
• This is where most of the work of being a cell lies
– During division the energy of the cell is devoted to making a new cell
– Death
• This can be a normal process creating a final functional form or an induced
suicide
– Epithelium and reticuloendothelial cells undergo active transitions towards
terminally differentiated states in which the final forms are unable to divide
» The stratum corneum consists of cells that have become bags of
crosslinked keratin protein with no internal metabolism
– Suicide can be induced because the organism senses a threat to the entire
organism
» Infection, cancer, avoidance of autoimmunity
Control of entry into cell cycle
and apoptosis
• Cell cycle is initiated by
phosphorylation of
transcription factors
• These activate
transcription of a set of
proteins known as cyclins
• The appearance of cyclins
is progressive and
determines the types of
proteins that will be
phosphorylated at a
particular point during the
cell cycle
Cyclins and CDK’s
• CDK levels don’t
change while cyclins are
destroyed at the end of
each phase
• There are 3 general
groups of each
– G1 cyclins
• Cyclin D
– S-phase cyclins
• Cyclin A
– G2 cyclins
• Cyclin B (maturation
promoting factor MPF)
– Cyclin E is shared
between G1 and M phase
– Cyclin A is shared
between M phase and G2
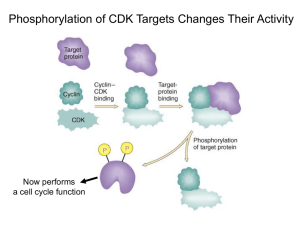
Cyclins bind
CDK’s
• CDK’s are Cyclin
Dependent Kinases
• Association with cyclins
activates their kinase
function
– A cyclin tethers a target
protein to the CDK
• The targets of CDK’s are
transcription factors among
other proteins
– CDK’s are serine/threonine
kinases
The exit from Go
• Go is a quiescent state
• Activation of G1 CDK
occurs due to a rising
level of G1 cyclins
• G1 cyclins are
transcriptionally
activated by growth
factors
Events during G1
• A rising level of G1
cyclins increases the
activity of G1 CDK’s
• CDK’s in turn activate
proteins and in turn genes
that prepare the cell to
begin DNA replication
• At the G1 S boundary, the
cell encounters a
checkpoint
• This is controlled by the activity of the
transcription factor E2F
– E2F is a family of related proteins (E2F 1 to
E2F5)
• E2F is found complexed throughout the
cell cycle to another family of proteins:
Rb
G1/S
checkpoint
– At the G1/S checkpoint, Rb is
phosphorylated by CDK2/cyclinA
– E2F is freed from sequestration and activates
transcription at genes containing an E2F
consensus sequence
And those genes are
• Three groups
– Cell cycle regulators
• Cyclin A
• E2F, Rb, myc, myb
– Note that these are not all
positive regulators of cell
cycle
– Enzymatic machinery for
DNA synthesis
• DNA polymerase
• PCNA
• Enzymes involved in
nucleotide metabolism
– DNA synthesis regulators
• Enzymes that recognize the
origins of replication for
example
Other Checkpoints
• These monitor the completion
of DNA synthesis
– The presence of Okazaki
fragments prevents entry into G2
• DNA damage
– This occurs before, during and
after completion of S phase
• Spindle attachment
– Failure to attach spindle to
centromere results in blockage of
mitosis at metaphase
– Failure to align the spindle during
cytokinesis results in blockage at
anaphase
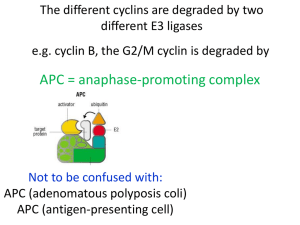
Downregulation of •
cyclin influenced
CDK activity
This is accomplished through
proteolysis of the cyclins
– G1 phase cyclins disappear during S
and G2 phase
– M-phase promoting factor (CDK2 +
cyclin B) concentrations rise just
prior to onset of mitosis
• Cyclins associated with MPF are
degraded by anaphase promoting
complex
Newly synthesized proteins labeled with 35S-methionine:
Mitosis
Interphase
Mitosis
Interphase
– Cyclin B levels peak at G1/M
» Degradation during anaphase
– APC promotes polyubiquitination
of cyclin B
– Ubiquitinated cyclin B is degraded
by a proteosome
• Cyclin transcription is also turned
off and the mRNA is unstable
cyclin A
cyclin B
ribonucleotide
reductase
Time
– So no new cyclin is made until
transcription is restored
MPF activates
APC which
ubiquitinates
cyclin B
In the overall
• Stimulated entry into G1
results in appearance of an
initial level of cyclins that
promote the progressive
activation of genes enabling
the cell to synthesize DNA
• A series of progressive
steps result in
– Activation of genes further
into the cycle
– Degradation of cyclins that
promoted earlier steps
– Passage through checkpoints
that insure mechanistic
fidelity of each step
Apoptosis
(apo – toe – sis)
• This is programmed cell death
– Distinguish it from necrosis
– Necrosis results from traumatic
forces outside the cell
– Necrotic tissue provokes
inflammation as the immune
system moves in to clear out
damaged and dead cells
• Apoptosis is an ordered
stepwise self-destruction that
permits surrounding cells to
utilize the breakdown products
of the dead cell
– There is no inflammation involved
The apoptotic cell
• Mitochondria break
open
• DNA fragments in a
regular way
• The cell loses a
regular shape
– Undergoes blebbing
– This is an irregular
bubbling appearance of
the plasma membrane
The mechanisms of apotosis
• Can be classified as
externally or internally
signaled
• One internal route
involves p53
• p53 is a transcription
factor that is involved in
cell cycle control and
sensing the presence of
DNA damage
• The central role p53 plays
is at the G1/S checkpoint
P53 controls entry into S-phase
• P53 can sense DNA damage by binding mismatches
• In the presence of damage, p53 activates transcription of p21
–
–
–
–
P21 binds and inactivates CDK2-cyclin E complexes
The complex is unable to phosphorylate Rb and free E2F
Thus entry into S phase is inhibited
If the damage is repaired, p53 levels and p21 levels drop and S phase ensues
But if the DNA damage is
extensive
• P53 induces apotosis by activating
transcription of Bax
– BAX protein competes with BCL-2 to form
pores in mitochondrial membranes
• BCL-2 prevents the release of cytochrome c from
mitochondria into the cytoplasm
• BAX permits release of cytochrome c
– When released, cytochrome c stimulates
caspase activation
The caspases
• These are proteolytic
enzymes that are held in
check by external or
internal inhibitors
• Activation results in an
explosive proteolytic
cascade
– Caspase 9 cleaves and
activates other caspases
– The caspases also activate
quiescent nucleases
External apoptotic mechanisms
• Involve external “death
signals”
• Cells may be recognized
as a threat to the whole
organism
– The immune system moves
in to kill them
– One mechanism of killing
involves a command to the
cell to initiate apoptosis
Fas/Fas ligand
signaling
• Fas ligand (FasL) is a
membrane bound cell
surface protein
• It binds to Fas receptor
• Binding results in
trimerization and
activation of APAF
• APAF in turn activates
caspase 8 by proteolysis of
a caspase 8 zymogen
– Caspase 8 cleaves a BCL-2
family member BID
– BID translocates to the
mitochondria and binds
BAX
– Bax permits leakage of
cytochrome c and activation
of the caspase 9 cascade via
APAF-1 again