2005 Images SC 1 to 4 - Cancer Insights at ASU
advertisement

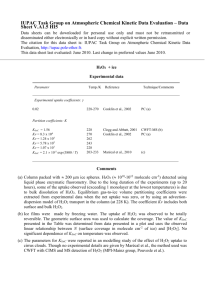
Workshop Summary and Clinical Implications Sidney M. Hecht, Director Center for BioEnergetics Biodesign Institute Arizona State University Mitochondria, Metabolism and Cancer Workshop March 23, 2012 Mitochondria in Health & Disease • Mitochondria have about 1600 imported gene • products ppppppp - about half have specialized functions and are organ-specific - functions include lipid metabolism, signal transduction • Clinical expression of mitochondrial disease requires a high level of mutations • Mitochondria are essential for nuclear DNA synthesis, and hence for tumor cell growth • Mitochondrial DNA fragments can be inserted into nuclear genes Cancer as a Metabolic Disease • Cancer results from damage to cellular respiration - many types of damage produce a similar response • Tumor cells produce most of the ATP from Gln by a compensatory fermentative process • Caloric restriction is associated with reduced blood glucose and increased ketone bodies • Caloric restriction leads to reduced tumor growth, is strongly antiangiogenic and pro-apoptotic and targets NF-kB-mediated inflammation • by Mitochondrial Dynamics • Primary mitochondrial disorders can be caused by any of about 240 mutations - ROS, mitochondrial membrane potential • There are also secondary mitochondrial disorders - [ATP], redox state, PTP, signaling • Mitochondria within the same cell can have different ATP levels and mitochondrial membrane potential • Nature of mitochondrial network (fragmented, dynamic, hyperfused) may control metabolic activity Mitochondria, Oxygen Sensing and Cancer • The mitochondrion is an oxygen sensor - in different cell types there may be very different responses to oxygen sensing by mitochondria • HIF-1a may be a key player in altering cells even without frank hypoxia • Cancer cells have increased membrane potential, and this is reversed by DCA, which induces apoptosis (via pyruvate dehydrogenase) • DCA decreases vascular perfusion in tumors • Mitochondrion has symbiotic relationship with endoplasmic reticulum Silencing Mitochondrial Genes • Mitochondrial DNMT1 leader peptides direct GFP to the mitochondria • Mitochondrial DNA contains both 5mC and 5hmC modifications • Loss of p53 preferentially upregulates the mitochondrial isoform of DNMT1 • Mitochondrial transcription patterns change in a gene specific fashion with an increase in mtDNMT1 • mtDNMT1 expression is regulated by factors that respond to oxidative stress; the mediators are NRF1 and PGC1a, which interact with the DNMT1 promoter • 2-HG is a competitive inhibitor of TET enzymes and histone demethylases Bioenergetics • Cancer cells have altered glycolysis and respiration • Cancer cell mitochondria support cell proliferation rather than efficient ATP production • Key factors for killing mitochondria - slow glycolysis - block lactate dehydrogenase/stimulate purvate dehydrogenase to promote TCA cycle and OXPHOS - target VDAC to form apoptosome - block NADH to deplete ROS scavenging systems - promote Ca2+ loading (block Na+/Ca2+ exchange) Oxidative Stress and Cancer • Animal models in which antioxidant systems have been knocked out exhibit enhanced age-related cancer development • Malignant tumors often have increased levels of DNA base oxidation • p53 has 10 cysteine residues and has been observed to be nitrated in human gliomas p53 Protein Has Both Pro- and Antioxidant Functions Production of Reactive Oxygen Species by Human Tumor Cell Lines • Constitutive H2O2 (but not superoxide) production has been observed for several cancer cell lines • Sustained increase in H2O2 shown to be essential to maintain transformed state • Inhibitors of mitochondrial respiration had no effect on H2O2 production • Cancer cells have enhanced resistance to H2O2-induced cell killing, even in the absence of catalase Strategies for Antitumor Therapy Based on Elevated Peroxide Levels in Cells • Suppress H2O2, causing a reversion of transformed phenotype • Enhance ROS, potentially disadvantaging tumor cells which already contain elevated H2O2 • Use elevated H2O2 to selectively activate toxins in tumor cells Activation of Prodrug with the Generation of a Quinone Methide and Ferrocenium Ion Mitochondrially Targeted Compounds H N H N O OCH3 H3CO N+ +HN NH+ ditercalinium P+ CH3O N+ CH3O mitoQ10 O Non-Peptidic Oligoguanidinium Vectors that Selectively Internalize Into Mitochondria Internalization of Oligomers 1 and 2 by HeLa Cells Cytotoxicity of Oligomers 1 and 2 Kinetics of Uptake of 0.5 mM Oligomer 1 Into HeLa Cells Distribution of Oligomer 1 and Colocalization with MitoTracker Orange Hydrogen Peroxide Sensing • Involves proteins that react with H2O2 faster than its reaction with glutathione • Enzymes that process H2O2 (peroxidases, catalase) have any of several reactive groups (Cys-thiolate, SeCys, Fe heme, vanadate) • Proteins modified by H2O2 almost all involve acidic Cys-thiolates • Protein modification occurs at 1-700 nM intracellular [H2O2], below levels associated with redox stress General Reaction of a Cys-thiolate Containing Protein with H2O2 Comparison of the Rates of Activation of OxyR with the Rates of Deactivation of Three Mammalian Proteins by H2O2 Cellular Proteins of Interest in Aging and Disease that are Under Redox Control • Nox1 (NADPH oxidase homologue) - overexpression can result in transformation of NIH3T3 cells - overexpression increases basal H2O2 levels 10-fold - sustained increase in H2O2 shown to be essential to maintain transformed state • Apoptosis-inducing factor (AIF) - present in mitochondrion and normally assists in radical and H2O2 scavenging - translocation outside mitochondria can trigger cell death - AIF deficiency associated with neurodegeneration Cellular Proteins of Interest in Aging and Disease that are Under Redox Control • Phosphatases (PTPs, PTEN, Cdc25c) - contain reactive active site cysteines that are easily oxidized to sulfenic acids - cysteine oxidation can alter kinase/phosphatase balance • p66Shc - mice lacking p66Shc have increased life span - cells from p66Shc-/- mice have reduced ROS levels • C-Myc - forced expression of c-Myc raises ROS levels - SOD2 levels are suppressed; peroxiredoxin 3 is increased Reversible Redox-Dependent Signal Transduction A Model for Oxidative Stress in Tumor Formation