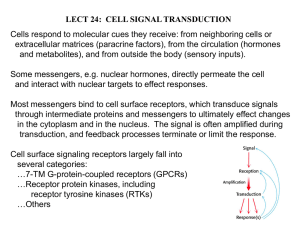
Ligand Gated Ion Channel
advertisement
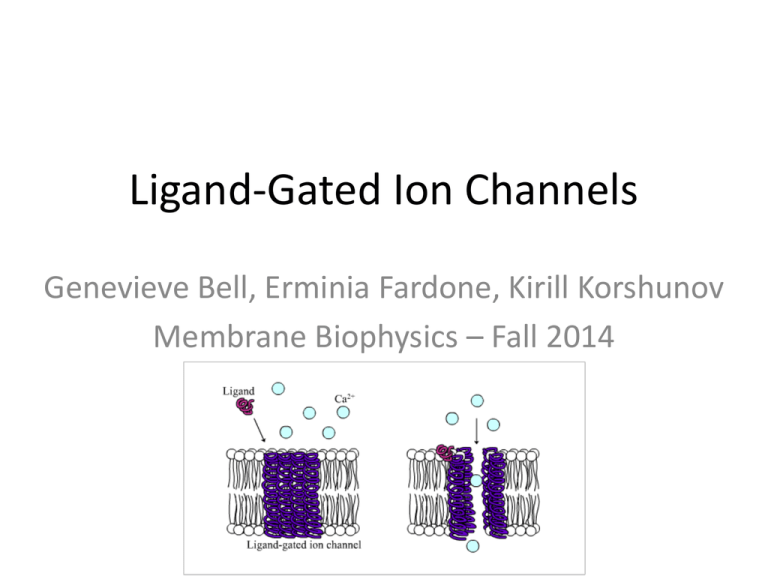
Ligand-Gated Ion Channels Genevieve Bell, Erminia Fardone, Kirill Korshunov Membrane Biophysics – Fall 2014 Tertiary Primary Secondary Quaternary Na+ L+R Na+ Na+ L+R LR Na+ O Na+ Na+ L+R LR Na+ O Na+ Na+ L+R LR Na+ D O L+R LR D 100 µM Zinc 50 µM AMPA 20 pA 5s 100 µM Zinc 50 µM AMPA 50 pA 5s 50 µM AMPA 100 µM Zinc 50 pA 5s Control Zinc Control Threshold for action potential Zinc Control Threshold for action potential Zinc Control Introduction • Phosphorylated Ligand Gated Ion Channels (pLGICs) include: – nAChRs – GABAARs – GlyRs – 5-HT3Rs • Best known for mediating fast neurotansmission in nervous system Introduction • Phosphorylation is well known to influence synaptic function by directly modulating pLGICs – Implicated in various disorders and can elicit a wide variety of effects – Understanding the structural basis of these effects design of specifically targeted drugs to treat pathological receptor modification pLGIC Architecture • Pentameric assemblies of identical or different subunits • 5 subunits together form a central water filled pore • Each subunit can be divided into three domains • Transmembrane α-helices form concentric rings around a central pore, directly lined by 5 M2 helices M3-M4 Cytoplasmic Domain • M3-M4 domain is poorly conserved in length and AA sequence and therefore exhibits structural variation • Interactions between M3-M4 loops and other proteins or ions are known to modulate pLGIC activity, assembly, and trafficking • M3-M4 domain is the only region known to house phosphorylation sites Receptor Phosphorylation Biological Function Chronic Inflammatory Pain • The α3-glycine receptor (α3 GlyR) is prominent in the spinal cord – Lamina I and II nociceptive neurons – Phosphorylation at serine346 attributed to chronic inflammation α3-Glycine receptor Mechanism of Inflammation Sensitization • Prosteglandin 2 (PGE2) activates PGE2 receptor • PGE2 stimulates adenylyl cyclase to produce more cAMP • cAMP activate cAMP-dependent protein kinase A (PKA) • PKA phosphorylates ser346 residue, causing a block in the IPSCs produced by glycine • Ultimately, this leads to a sensitization in nociception in the spinal cord lamina I and II neurons Zeilofer HU., 2005 Other Disorders Implicated in Phosphorylation of pLGICs • Alcoholism – Implicated in GABAARs • PKC inhibits GABAARs IPSCs • Nicotine addiction – Implicated in α4β2 nAChRs • Phosphorylation/dephosphorylation lead to receptor desensitization at the Ser368 residue • Continuous nicotinic exposure leads to permanent receptor desensitization • Myasthenia gravis – Implicated in muscle AChRs • PKA phosphorylates γ and δ subunits • PKC phosphorylates α and δ subunits • PTK phosphorylates β, γ, and δ subunits Summary • Nociception sensitization occurs in α3 GlyRs, caused by phosphorylation of the ser346 residue – This is a possible mechanism for chronic inflammation • Alcoholism is attributed to GABAAR phosphorylation • Nicotine addiction is attributed to desensitization of α4β2 nAChRs – Continuous nicotinic exposure leads to permanent channel desensitization • Phosphorylation of different muscle AChRs subunits could lead to myasthenia gravis Possible medical application Inappropriate phosphorilation Global allosteric conformational change Neurological disorders Chronic pain: PKA-mediated phosphorilation α3 GlyRs inhibit current and causes a conformational change of the gly-binding site. Alcholism: Protein phosphorilation can casue an increase in ethanol sensitivity of γ2containing GABAARs. Conformational changes in pLGICs can be targeted by drugs to treat several diseases. GABAA Receptor α and γ Subunits Shape Synaptic Currents via Different Mechanisms Christine Dixon, Pankaj Sah, Joseph W. Lynch, and Angelo Keramidas Queensland Brain Institute, University of Queensland, Australia Introduction GABAA Receptors - Mediate the majority of inhibitory neurotransmission in the mammalian brain - Pentamers : Consist of two α, two β, and a γ subunit - 6 different α subunits - 4 different β subunits - 3 different γ subunits - GABAA R that contain a variety of subunits are expressed throughout the brain Inhibitory Postsynaptic Currents - IPSCs at GABA-ergic are determined by: • The biophysical properties of postsynaptic receptors • How receptors are clustered at the postsynaptic membrane - α subunit = Key determinant of the functional properties of GABAA R The Amygdala • Plays a key role in processing fear • Dysfunction associated with anxietyrelated disorders • Disorders are typically managed via benzodiazepines Benzodiazepines • Enhances the action of GABA at GABAA R containing γ2 subunits • Acts indiscriminately on GABAA R throughout the brain, producing side effects such as tolerance and sedation α1 and γ2 subunits are expressed throughout the CNS, while the α2 and γ1 subunits have restricted distribution : • • • • • • Amygdala Forebrain Cerebellum Hypothalamus Pallidum Substantia Nigra Properties of receptors containing α1 and γ2 subunits and their impact on synaptic currents are very well known, in contrast nothing is known regarding the impact of γ1 containing GABAA R on inhibitory synaptic current Experimental Procedures • Cell Culture and Molecular Biology • Subunits were transfected into HEK293 cells • Primary neuronal cell culture • Immunofluorescent Labeling • Electrophysiology • Patch-clamp: Outside-out and macropatch Effects of Zn2+ on wild and mutant neuronal α7 nicotinic receptors E. Palma, L. Maggi, and F. Eusebi PNAS 1998 Introduction • α7 nAChR is a ligand-gated ion channel largely present in the hippocampus and the retina. – Receptor dysfunction linked to epileptic seizures and schizophrenia. • A mutated form of α7 (L247Tα7) exhibits spontaneous inward currents in the absence of ACh. • Zn2+ is also largely found in the hippocampus and retina. – How does Zn2+ affect α7 nAChRs? – Influence on the spontaneous currents? α7-nicotinic acetylcholine receptor Methods and Materials • Model organism = Xenopus oocytes. • cDNA (for either WTα7 or L247Tα7) was injected into the nuclei of stage 6 oocyte. • Electrophysiology – – – – – Two-four days after cDNA injection Voltage-clamp ACh applied at 3 min intervals Zn2+ from ZnCl2 and Zn2+ acetate nAChR blockers methyllycaconitine (MLA) and αbungarotoxin (Bgt) Zinc’s effect on WTα7 ACh current (IACh) Zn2+ Blocks WTα7 IACh [Zn2+] = 10 nM to 10 mM [ACh] = 150 μM Zn2+ Blocks WTα7 IACh VoltageIndependently [Zn2+] = 20 μM [Zn2+] = 30 μM [ACh] = 150 μM L247Tα7 and the zinc current (IZn) Zn2+ Induces Currents in L247Tα7 [Zn2+] = 1 mM [ACh] = 0.2 μM [MLA] = 1 μM [αBuTx ] = 100 nM The Dual Role of Zn2+ on L247Tα7 [Zn2+] = 10 fM to 10 mM IZn on L247Tα7 Mimics IACh on WTα7 IACh on WTα7 @ +45 mV @ -100 mV [Zn2+] = 10 nM L247Tα7 has one Zn2+-Gated Channel Pore @ - 63 mV [Zn2+] = 10 nM Zn2+-induced Modulation of IACh in L247Tα7 Zn2+ Modulation of IACh in L247Tα7 For A + C: [Zn2+] = 1 mM For B: [Zn2+] = 10 mM Conclusion WTα7 – Pretreatment (20-30 s) of Zn2+ blocks IACh – Blockage increases with [Zn2+] – Blockage is voltage-independent L247Tα7 – Zn2+ produces its own current (IZn) – Zn2+ acts as an agonist at low concentrations (10 fM10 nM) – Acts as an antagonist at higher concentrations (10<). • Voltage-dependent when co-applied with ACh. – Zn2+ activates one open state The tetrameric structure of a glutamate receptor channel Rosenmund C, Stern-Bach Y, Stevens CF (1998). Science 280: 1596-1599. Backgroud • The AMPA-type glutamate receptor (AMPAR) is widely expressed in the brain and mediates the majority of fast excitatory neurotransmission. • The AMPAR is a transmembrane glutamategated ion channel comprised of 4 pore-forming subunits GluA1–4. Methods used cDNA Expression and Cell Culture: HEK (Human embryonic kidney)cell line transfected with a-amino-3hydroxy-5-methyl-4-isoxazol propionate (AMPA)– receptor. - Receptor subunits: GluR6/GluR3 - Alternative splice variant: GluR3flip Patch-clamp recording: outside-out patch - AMPA receptor agonist: quisquillate (QUIS) - AMPA receptor antagonist: 2,3-dihydroxy-6nitro-7-sulfamoyl-benzoquinoxaline (NBQX) Foundings (1) Saturating agonist concentrations (1 mM) consistently caused a noninactivating channels to open state Foundings (2) Agonist-binding sites were presaturated with the competitive antagonist, NBQX, before agonist (QUIS) application, so that each agonist-binding site was only made available after an antagonist molecule dissociated from the receptor. NBQX: 10 to 30 µM C= Closed state Staircase fashion: S= Small, 5 pS* M= Medium, 15 pS L = Large, 23 pS *pS=picoSiemens (electrical conductance) Foundings (3) No artifacts of GluR3/GluR6 following the sensitivity to allosteric modulator (Cyclothiazide) with flop splicing variants of GluR3 receptors 100 µm cyclothiazide to remove the inactivation Foundings (4) Activation of single receptor/channels proceeds through the staircase of openings to three different conductance levels of increasing amplitude Waiting times reveal 4 subunits Conclusion The authors proposed a model where each receptor contains four functional antagonist/agonist-binding sites, which is consistent with a tetrameric protein. Outside-out patch






![Shark Electrosense: physiology and circuit model []](http://s2.studylib.net/store/data/005306781_1-34d5e86294a52e9275a69716495e2e51-300x300.png)