MacroMolecules
advertisement
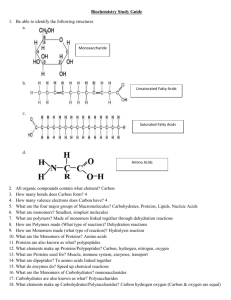
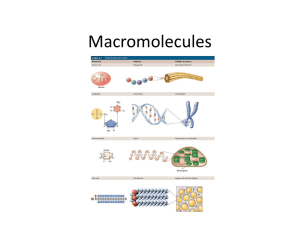
Macromolecules Introduction • Organic Compounds – contain carbon and hydrogen atoms • Inorganic Compounds – contain one or the other, but not both. • Most of your body’s molecules are organic. Macromolecules • Built from small organic compounds by linking a lot of chains Monomers • Large carbon compounds are built up from smaller simpler molecules. Mono = One Polymers • When monomers bind to one another to form a complex molecule. • Poly = many • Consists of repeated linked units which bind forming Macromolecules. • Macro = large Chemical reactions • Monomers link to form polymers through a chemical reaction called condensation reaction or dehydration synthesis. • Water is released or is a byproduct of the reaction. Hydrolysis • Break down of some complex molecules • Hydrolysis is the reverse of a condensation reaction. 4 main types of macromolecules • • • • 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Carbohydrates • Composed of carbon, hydrogen, and oxygen atoms in the proportion of 1:2:1 • Glucose formula: C6H12O6 • SHORT TERM ENERGY • MAIN Source of Energy Monosaccharide's • Simple sugars Examples 1. Glucose: found in blood of animals 2. Galactose: found in milk 3. Fructose: found in fruit Isomers – same formula, but different structure Disaccharides • Contain 2 monosaccharide's joined by dehydration synthesis. Examples 1. Lactose: found in milk 2. Sucrose: transported in plants Polysaccharides • Carbohydrates formed from linking individual sugars into long chains. Examples 1. Starch: storage of glucose in in plants 2. Cellulose: contained in cell walls of plants 3. Glycogen: storage of glucose in animals (stored in the muscles and liver) Lipids • Do not dissolve in water 3 Functions 1. Energy storage – LONG TERM ENERGY 2. Structural support for cell membranes 3. Serve as a reactant (starting material) for metabolic reactions Lipids Cont. • Phospholipids make up the cell membrane. Fatty Acids • Building blocks that make up most lipids • Classified as either saturated or unsaturated Saturated Fatty Acids • • • • Have the maximum number of bonds possible They are full Usually solid at room temp Most come from animal products Unsaturated Fatty Acids • Have double bonds in the carbon chain • Most are liquid at room temp • Usually referred to as oils Triglycerides • Tri = 3 • Common lipid that contains fatty acids • Glycerol linked to 3 fatty acids in the shape of an E by condensation reaction. Proteins • Composed mainly of carbon, hydrogen, and nitrogen atoms • Construction materials for body part like hair, skin, nails, and blood Amino Acids • The building blocks that make up most proteins. • 20 different kinds of amino acids Enzymes • Important group of proteins • Help control chemical reactions by acting as catalysts. • Catalysts speed up reactions by lowering the activation energy. • Enzyme rates are affected by Ph, hot and cold temperatures. Enzymes - Add • Substrates – reactants of enzyme – catalyzed reactions. • Reduces the energy needed for the reaction. • Works like a lock and key. VERY IMPORTANT • Proteins DO NOT produce, store, transmit, or have anything to do with ENERGY. • Do not get confused with protein bars!!! Nucleic Acids • Complex organic molecules that store genetic information in the cell. Nucleotides • Nucleotides are the building blocks that make up most nucleic acids • Consist of sugar, base, phosphate 3 main types of Nucleic Acids • 1. DNA – Deoxyribonucleic acid – Genetic info inside the nucleus of cells 2. RNA – Ribonucleic acid - code for protein synthesis 3. ATP – Adenosine Triphosphate - Contains a base, sugar, and 3 phosphates - ATP is used as energy for the cell