MET - Imedex
advertisement

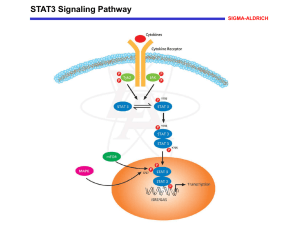
The MET oncogene: From molecular biology to Clinical Trials Paolo M. Comoglio, MD Institute for Cancer Research @ Candiolo, University of Turin, Medical School, Italy MET IS A ‘MASTER GENE’ IN THE CONTROL OF INVASIVE GROWTH HGF (Scatter Factor) Sema domain 500 AA MRS (PSI) G-P rich Ig like domains 400 AA Kringles Proteaselike P P Tyrosine Kinase P P Met (HGF Receptor) Plexin B1 MET Integrin 6 4 CD44v6 Link to cytoskeleton PI3K RAS P P p85 P P GRB2 STAT Transient MAP kinase activation (proliferation) p85 PI3K CRKL GAB1 P SHP2 GRB2P PLC SRC Cell-Polarity and Morphogenesis C.Boccaccio and PM.Comoglio. Nature Rev. Cancer. 2006, 6: 637-645. Sustained MAP and PI3 kinase activation (invasion / apoptosis protection) MET IS A FUNCTIONAL MARKER OF CANCER STEM CELLS Human metastatic colorectal cancer Xenopatient Spheropatient Xenosphere (cancer stem cells) Xenopatients, Xenospheres and Spheropatients Xenopatient Xenosphere (cancer stem cells) Spheropatient (Secondary xenopatient) Mutations retained and passed on Response to targeted drugs retained and passed on C. Boccaccio et al., in preparation MET inhibition promotes differentiation of CRC colospheres Control JNJ inhib. Cetuximab Fibroblast conditioned medium 0% serum ACTIVATION OF THE MET-DRIVEN ‘INVASIVE GROWTH’ PROGRAMME GENERATES AN AGGRESSIVE PHENOTYPE • ONCOGENE ‘EXPEDIENCE’ • ONCOGENE ‘ADDICTION’ THE CONCEPT OF ‘ONCOGENE EXPEDIENCE ‘ • Met can promote invasion and metastasis independent of the genetic lesions that caused tumour onset • Met activation is a ‘stress’ response to unfavourable microenvironment (hypoxia, radiations , inflammatory cytokines) • Met activation occurs mainly through transcriptional upregulation • Met activation is an expedience that provides a pro-survival and invasive advantage Trusolino and Comoglio., Nature Rev. Drug Discov, 2008 Nature Rev. Mol. Cell. Biol. 2010 Hypoxia promotes invasive growth by transcriptional activation of the MET Oncogene Cellular [O2] Proteasome degradation HIF Proline Hydroxylase pVHL Cytoplasm HIF-1 (regulated) -OH Nucleus HIF-1 HIF-1 (constitutive) P (-2619) S (-411) S (-345) A (-253) HRE-1 HRE-2 S (-295) A (-32) START S (+89) AP-1 asHRE-1asHRE-2/ HRE-3 The MET promoter HRE-4 A (+353) HRE-5 CoCl2 MET 3% O2 21% O2 Control Hypoxia promotes invasive growth by transcriptional activation of the MET Oncogene met mRNA HIF-1a 28S Pennacchietti, Michieli et al. , Cancer Cell 3: 347 (2003) THE CONCEPT OF ‘ONCOGENE ADDICTION ‘ • In a limited number of cancer types, genetic lesions of Met are selected along the tumour natural history to become the driving force that maintains the transformed phenotype • Met activation is the consequence of a fixed and transmissible genetic alteration; therefore, it is characterized by chronic firing and high steady-state signalling ‘Oncogenic addiction’ to MET in tumours can be achieved by: • Amplification (e.g. gastric ca, NSCLCs and CRC mts resistant to targeted therapy) • Point mutations - hereditary and sporadic carcinomas (e.g. kidney, hepatocellular, head & neck ca. - early metastatic cancers of unknown primary origin (CUPs) Trusolino and Comoglio, Nature Rev. Drug Discov. 2008 TARGET THERAPIES AGAINST MET • An adjuvant approach for a vast number of tumours: Overexpression: targeting ‘expedience’ (almost all solid tumors expressing MET) - Endpoints: PFS, OS • A front-line intervention for a limited fraction of genotype-specific tumours: Amplification, mutations : targeting ‘addiction’ (e.g. gastric ca., NSCLC, gefitinib-resistant NSCLCs) - Endpoints: Remission THERAPEUTIC INACTIVATION OF THE MET ONCOGENE CAN BE ACHIEVED BY: 1. Small molecules inhibiting the Tyrosine Kinase 2. Monoclonal Antibodies RESPONSE TO MET INHIBITORS CORRELATES WITH GENE AMPLIFICATION 200 cancer l. tested <3% ADDICTION 97 % EXPEDIENCE Cell Line MET copy N° EBC-1 5.8 MKN-45 6 GTL-16 6.1 HS746T 6.3 SNU5 5.6 NCIH1993 5.2 Hypoxia (or HGF) PHA-665752 PHA-665752 Clinical trials for MET inhibitors • Inhibition of growth (reduction of tumor mass) is expected only in cancers “addicted” to MET • Genetic lesions are expected in ~ 3-4 % of epithelial cancers • It is mandatory to identify patiens with cancers “addicted” to MET before recruitment 111In DTPA-DN30 tumor uptake at 2h post injection SPEC-CT scan A2780 EBC1 Bardelli. et al. Cancer Discovery 2013 Detection of MET-amplification by Liquid Biopsy cell viability % Xenopatient-derived cancer cells display MET amplification and are sensitive to Met targeted drugs 1,4 1,2 1 0,8 0,6 0,4 0,2 0 NT 10 50 JNJ 500 10 50 500 CRIZOTINIB Therapy with MET antibody MV-DN30 Monoclonal Antibody Monovalent, Chimeric, Stabilized O OH n • • • • Recombinant, properly assembled and PEGylated Bind Met (IP4) with high affinity (Kd= 0,116 nM) Down-regulate the Met receptor from the cell surface Induce shedding of the extracellular domain (“decoy”) Ligand neutralization Inactive receptor heterodimer p125 Decoy Inhibitory effects Shedding Adam 10 Γ- secretase Proteasome degradation Met p175 p55 p50 Exogenous administration of anti-MET DN-30 inhibits tumor growth and prevent metastasis Human Breast Carcinoma, transplanted in Athymic nu/nu mice Irrelevant Mab Mab DN30 Petrelli, A. et al., Proc. Natl.Acad Sci. US: 2006, 28, 5090-5095 CHEMOTHERAPY (CDDP, 5FU) vs MET ANTIBODY TREATMENT OF EBC1 - Xenotransplants2200 NT CDDP 2000 5FU MV DN30 CDDP + MVDN30 1800 5FU + MVDN30 1400 Tumor volume (mm3) Tumor Volume . 1600 1200 1000 800 600 400 200 0 0 2 4 6 8 10 12 14 16 18 Days after injection Days after injection 20 22 24 26 28 MECHANISMS OF ACQUIRED RESISTANCE TO MET KINASE INHIBITORS • MET amplification • Activating point mutations Amplification of MET contributes to acquired resistance to MET kinase inhibitors Wt PHA resistant (150 nM) Chromosome 7 centromere GTL16 MET amplicon marker wt Some MET mutations confer resistance to MET inhibitors PHA 250 nM M1131T V1188L L1196V V1220I P S985 P Y1003 P P D1228H (Kit) D1228N (Kit) Y1230C Y1230H M250T (Ret) a-pMet a-Met P Y1349 P Y1356 FAb DN30 treatment overcomes resistance to the MET kinase Inhibitor PHA-665752 Viability in 50 nM PHA DN30 Fab 0.4 mM EBC1 wt EBC1 Wild Type EBC1 PHA Resistant EBC1 PHA Resistant MET and Invasive Growth: State of the art: 1. Scatter factors and the receptors of the MET oncogene family (RON, ROR) are key regulators of the Invasive Growth program in cancer stem cells. 2. The program is activated by MET over expression, induced by unfavourable micro-environmental conditions, such as hypoxia or ionizing radiations: (“Oncogene Expedience”). 3. The invasive growth program is constitutively activated in some cancers, by MET amplification, mutations or autocrine loops (“Oncogene Addiction”). 4. The invasive growth program driven by the oncogene MET is a good target for therapy. 5. Resistance may occur, that may be overcome by combined treatment with s.m. inhibitors and antibodies IRCC Experimental Clinical Molecular Oncology Met and resistance S. Giordano S. Corso A. Petrelli C. Migliore Met & Stem cells C. Boccaccio F. De Bacco P. Luraghi G. Reato Ron & ROR S. Benvenuti A. Gentile L. Lazzari A. Arnesano Met & Hypoxia P. Michieli S. Pennacchietti C. Basilico Met Addiction L.Trusolino A. Bertotti F. Galimi Met Gene Therapy E. Vigna S. Cignetto R. Albano Met Signature E. Medico Met Biology L. Lanzetti Met Genetics A. Bardelli M.F. Di Renzo Plexins L.Tamagnone E. Giraudo G. Serini “Today Science, Tomorrow Medicine” IRCC: Institute for Cancer Research @ Candiolo