Lecture 1: Introduction and review
advertisement

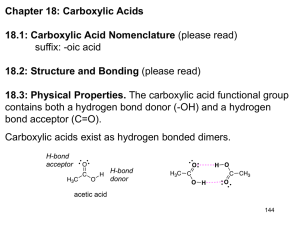
Lecture 1: Introduction and review – – – – – – Quiz 1 Website: http://www.esf.edu/chemistry/nomura/fch530/ Review of acid/base chemistry Universal features of cells on Earth Cell types: Prokaryotes and Eukaryotes Quiz Friday on the 20 amino acids-you need to know the structures, names, single letter codes, and pKa’s of the side chains Review of pH, acids, and bases • pH is generally defined as the negative logarithm of the hydrogen ion activities (concentration) expressed over 14 orders of magnitude pH = -log10 [H+] • • • The pH scale is a reciprocal relationship between [H+] and [OH-] Because the pH scale is based on negative logarithms, low pH values represent the highest [H+] and thus the lowest [OH-] At neutrality, pH 7, [H+] = [OH-] Review of pH, acids, and bases • • Strong electrolytes dissociate completely in water – Electrolytes are substances capable of generating ions in solution – Increase the electrical conductivity of the solution The dissociation of a strong acid in water H3O+ + Cl- HCl + H2O The equilibrium constant is K= [H3O+][Cl-] [H2O][HCl] [H2O] is constant in dilute aq. Solutions and is incorporated into the equilibrium constant. giving rise to a new term Ka-the acid dissociation constant = K[H2O], [H3O+] is expressed as [H+] [H+][Cl-] Because Ka is large for HCl, [H+] in solution = [HCl] added to solution. a K= [HCl] Thus, a 1M HCl solution has a pH of 0, a 1 mM HCl solution has a pH of 3, and so on. Conversely, 0.1 M NaOH solution has a pH of 13. Ka= [H+][Cl-] [HCl] For a strong acid Ka will approach be large because the nearly all of the protons will be dissociated. The [H+] at equilibrium is equal to the initial concentration of the acid. Calculate the pH of a 1M HCl solution HCl + H2O H3O+ + Cl- 0.0004% at equilibrium 99.996% at equilibrium Since we are at equilibrium, H3O+ is equal to the initial concentration of acid. [H+] = [H3O+] = [HCl] = 1M We know that pH is the -log of [H+], therefore for 1M HCl at equilibrium pH = -log10 [H+] pH = -log10 (1) pH = 0 [H+] pH [OH-] 0 (100) 1.0 0.00000000000001 (10-14) 1 (10-1) 0.1 0.0000000000001 (10-13) 2 (10-2) 0.01 0.000000000001 (10-12) 3 (10-3) 0.001 0.00000000001 (10-11) 4 (10-4) 0.0001 0.0000000001 (10-10) 5 (10-5) 0.00001 0.000000001 (10-9) 6 (10-6) 0.000001 0.00000001 (10-8) 7 (10-7) 0.0000001 0.0000001 (10-7) 8 (10-8) 0.00000001 0.000001 (10-6) 9 (10-9) 0.000000001 0.00001 (10-5) 10 (10-10) 0.0000000001 0.0001 (10-4) 11 (10-11) 0.00000000001 0.001 (10-3) 12 (10-12) 0.000000000001 0.01 (10-2) 13 (10-13) 0.0000000000001 0.1 (10-1) 14 (10-14) 0.00000000000001 1.0 (100 ) Review of pH, acids, and bases • • Weak electrolytes only slightly dissociate in water – Acetic acid, CH3COOH The dissociation of a weak acid in water CH3COOH + H2O H3O+ + CH3COO- The acid dissociation constant is Ka = [H+][CH3COO-] [CH3COOH] = 1.74 X 10-5 M Ka is also called the ionization constant, because Ka is small, most of the acetic acid is not ionized. Acid dissociation constant – The general ionization of an acid is as follows: HA H+ + A- So the acid dissociation constant is as follows: Ka = [H+][A-] [HA] There are many orders of magnitude spanned by Ka values, so pKa is used instead: pKa = - log10 Ka The larger the value of the pKa, the smaller the extent of dissociation. pKa <2 is a strong acid Henderson-Hasselbalch Equation • Describes the dissociation of a weak acid in the presence of its conjugate base – The general ionization of a weak acid is as follows: HA H+ + ASo the acid dissociation constant is as follows: Ka = [H+][A-] [HA] Rearranging this expression in terms of the parameter of interest [H+] gives the following: [H+] = Ka [HA] [A-] Henderson-Hasselbalch Equation Take the log of both sides: log[H+] = [HA] log Ka + log [A-] Change the signs and define pKa as -log Ka : pH = pKa - log [HA] [A-] or pH = pKa + log [A-] [HA] Titration curves and buffers • Titration curves can be calculated by the Henderson-Hasselbalch equation – As OH- is added to the reaction, it reacts completely with HA to form A- [A-] = x vol x = the equivalents of OH- added and V represents the volume of the solution. If we let co represent HA equivalents initially present, then: [HA] = (co-x) vol We can reincorporate this into the HendersonHasselbalch eqn. x (c -x ) pH = pKa + log o Buffers • • • • Buffers are solutions that resist changes in their pH as acid (H+) or base (OH-) is added. Typically, buffers are composed of a weak acid and its conjugate base. Acids = Proton (H+) donors Bases = Proton Acceptors HA Acid • • • • H+ + Aconjugate base Acids and their conjugate bases are in equilibrium. Equilibria are related to the properties of the reactants and products, so for weak acids, the tendency to give up its proton determines its buffering property + The tendency to ionize can be put in an equilibrium equation Ka= [H ][A ] [HA] A solution of a weak acid that has a pH near to its pKa has an equivalent amounts of conjugate base and weak acid. Typically a weak acid is in its useful buffer range within 1 pH unit of its pKa. Polyprotic acids • • • Have more than one acid-base group H3PO4 and H2CO3 The pK’s of two closely associated acid-base groups are not independent - the closer they are, the greater the effect. – Examples: oxalic acid and succinic acid OO H-O-C-C-O-H pK differs by 3 pH units O O H-O-C-CH2CH2-C-O-H pK differs by 1.4 pH units Polyprotic acids • • The effect of having successive ionizations from the same center is even greater. However if pK’s of polyprotic acid differ by less than 2 pH units, this reflects the average ionization of all of the groups. Universal features of cells • “Life possesses the properties of replication, catalysis, and mutability.” - Norman Horowitz • Life requires free energy. – Main energy currency is • ATP (bond energy, G) • NADH, NADPH (redox energy) – All cells obey the same laws of thermodynamics (see Ch.3). G (Gibbs free energy) must be negative (spent) S (Entropy) increases – Sources of energy may vary • Purple sulfur bacteria • Humans • Plants H2S CH2O h So H2O + CO2 Universal features of cells (cont.) • Most organisms are composed of only 16 chemical elements • (H,C,N,O,P,S,Mn, Fe, Co, Cu, Zn, Na, Mg, Cl, K, Ca). – Chemical makeup appears to be determined partly by the availability of raw materials and the specific roles of molecules in life processes. – Do not reflect the composition of the biosphere – Examples on per atom basis, H in organisms = 49%, H in Earth’s crust = 0.22 %, Si in organisms = 0.033%, Si in Earth’s crust = 28%) • H, O, N, and C, make up >99% by weight of living matter are the smallest atoms that can share 1, 2, 3, and 4 electrons respectively. • O, N, and C are the only elements that easily form strong multiple bonds. • O2 is soluble in water and readily available to all organisms. • Phosphorous and sulfur are unstable in the presence of water. – Require a large amount of energy to form. – Energy released when they are hydrolyzed. MW 18-44 N2, H2O, CO2 20 amino acids 100-250 100-800 Amino acids 104-109 106-1010 Proteins 5 aromatic bases, ribose Nucleotides Nucleic acids Glucose Palmitate, glycerol, choline Sugars Phospholipids Polysaccharides Multienzyme complexes, ribosomes, chromosomes, membranes, structural elements Organelles, Cells, Tissues, Organs, Organsims Universal features of cells (cont.) • All cells function as biochemical factories and use the same basic molecular building blocks. • Proteins (amino acids – – – – – – – • Can be structural or catalytic Enzymes Transport (Na+/K+ pump) Storage (ferritin) Signals (hormones/toxins), examples insulin or botulinum toxin Receptors Structure (collagen, elastin) Lipids (fatty acids – – – – – polypeptides lipids) Membranes Triglycerides (energy storage) Phospholipds (membrane structure) Sphingolipids (found in nerve cells and brain tissue) Sterols (hormones and membranes) proteins) Universal features of cells (cont.) • Carbohydrates – – – – Monosaccharides (glucose, fructose) Disaccharides (sucrose, maltose) Trisaccharides (raffinose) Complex carbohydryates • Starch (energy storage) • Cellulose (structure, cell wall) • Cell-cell recognition • Nucleic acids – DNA (genetic material) – RNA (mRNA, tRNA, pre-mRNA or hnRNA, rRNA) • Proteins and nucleic acids are produced by the same rules – Central dogma DNA RNA • A living cell can exist with fewer than 500 genes! Protein Types of cells • There are two major cell types: eukaryotes and prokaryotes. • Eukaryotes have a membrane enclosed nucleus encapsulating their genomic DNA. • Prokaryotes do not have a nucleus. Prokaryotes Eukaryotes Bacteria, Archaea Fungi, Protists, Animals, Plants 1-10 µm 10-100 µm General schematic for a prokaryote cell General schematic of an animal cell General schematic of a plant cell MW 18-44 N2, H2O, CO2 20 amino acids 100-250 100-800 Amino acids 104-109 106-1010 Proteins 5 aromatic bases, ribose Nucleotides Nucleic acids Glucose Palmitate, glycerol, choline Sugars Phospholipids Polysaccharides Multienzyme complexes, ribosomes, chromosomes, membranes, structural elements Organelles, Cells, Tissues, Organs, Organsims







![pH = - log [H + ]](http://s2.studylib.net/store/data/005622524_1-002df1ea50d2a849b15deb604928664e-300x300.png)