Dielectric Properties
advertisement

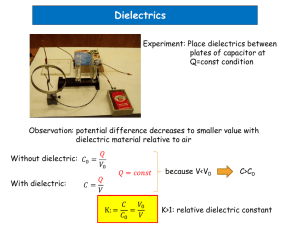
Dielectrics Unit – 1 Sub.- Physics Introduction Dielectrics are the materials having electric dipole moment permantly. Dipole: A dipole is an entity in which equal positive and negative charges are separated by a small distance.. DIPOLE moment (µele ):The product of magnitude of either of the charges and separation distance b/w them is called Dipole moment. µe = q . x coul – m q X -q All dielectrics are electrical insulators and they are mainly used to store electrical energy. Ex: Mica, glass, plastic, water & polar molecules… POLARISATION OF DIELECRICS When we are applying external electric field, it causes the electron cloud to move away. Thus the centroides of the positive and negative charges now no longer coincides and as a result of that an electric dipole is induced in the atom. Thus, atom is said to be polarized. Polarization : the process of creating or inducing dipoles in a dielectric medium by an external field. On the basis on that dielectrics are the material that have either permanent diploes or induced in the presence of external electric field . They are classified into two categories (1) Non polar (2)polar Dielectrics Non Polar Dielectrics : There is no permanent dipole existence in the absence of an electric field . Centroids of positive and negative charges of molecules constituting the dielectric material coincide . Examples :H2, N2, O2, CO 2 Polar Dielectrics : there is permanent dipole exists even in the absence of an electric field . Centroids of posistive and negative charges of molecules constituting the dielectric material do not coinside even in the absence of electric field Examples : HCL , CO dipole _ + Electric field + + + _ _ _ _ + + + _ _ + _ + _ Dielectric atom Dielectric Constant Dielectric Constant is the ratio between the permittivity of the medium to the permittivity of free space. r 0 its value changes widely from material to material For vacuum =1 For all other dielectric it is Ɛr >1. So, we can write Ɛr=1+χe , χe is susceptibility The characteristics of a dielectric material are determined by the dielectric constant and it has no units. Electric field The region surrounded by charged body is always under stress because of electrostatic charge . If a small charge q or a charged body is placed in this region ,then the charge q or a charged body will experienced a force according Coulomb s law . This stressed region around charged body is called electric field . Electric field at a point define as the force that acts on a unit positive charge placed at that point thus E. According to coulomb law when two point charges Q1 and Q2 are separated by a distance r, the force of attraction or repulsion between two charges is given by Electric Polarization The process of producing electric dipoles by an electric field is called polarization in dielectrics. Polarizability: The induced dipole moment per unit electric field is called Polarizability. The induced dipole moment is proportional to the intensity of the electric field. E E polarizability constant Electric flux ɸ (1) Number of lines of force that pass through a surface placed in the vector field . (2) As the product of surface area and the components of the electric field normal to the surface a unit charge is supposed to emanate one flux. In the case of an isolated charge q coulomb the flux is q=ɸ Is independent of of nature of medium. Polarization vector Definition: induced dipole moment per unit volume of dielectric medium. P is vector quantity and its direction is along the direction of applied field .If m is the average induced dipole moment per unit molecule and N is the number of molecule per unit volume then polarization is given by P=Nµ V=At ,where A is the area of slab Thus ,the polarization is also defined as induced surface charge per unit area But q/A is the induced charge density .so magnitude of polarization is equal to the induced charge density. Electric flux Density (D): Electric flux density is defined as charge per unit area and it has same units of dielectric polarization. Electric flux density D at a point in a free space or air in terms of Electric field strength is D0 0 E - - (1) At the same point in a medium is given by D E - - (2) As the polarization measures the additional flux density arising from the presence of material as compared to free space i.e, D 0E P - - (3) Using equations 2 & 3 we get E 0 E P ( - 0 ) E P (or) ( r . 0 - 0 ) E P ( r 1) 0 .E P Electric susceptibility The polarization vector P is proportional to the total electric flux density and direction of electric field. Therefore the polarization vector can be written as, In a large number of dielectrics it is Found that polarization is directly Proportional to the external applied Field E. P 0 e E P e 0E Def. The ratio of polarization to net electric field as 0 ( r 1) E 0E Given by the induced charge on the surface of e r 1 Dielectric is called susceptibility. Relation between polarization P, susceptibity χ and dielectric constant Ɛr Lets us consider a parallel plate capacitor between which an electric field Ɛ0. If σ is the surface charge density then gauss law. E=σ/Ɛ0 (1) if a dielectric slab is placed between the plates of capacitors , then due to polarization, charges appear on the two faces of the slab and establish another field E1 within the dielectric . This field will be in a direction opposite to that of E0 Resultant value E=E0-E1 (2) If σs is the surface charge density on the slab then from (1) E1= σs / Ɛ0 (3) From (1)(2)(3) Various polarization processes: When the specimen is placed inside a d.c. electric field, polarization is due to four types of processes…. 1.Electronic polarization 2.Ionic polarization 3.Orientation polarization 4.Space charge polarization Electronic Polarization When an EF is applied to an atom, +vely charged nucleus displaces in the direction of field and ẽ could in opposite direction. This kind of displacement will produce an electric dipole with in the atom. i.e, dipole moment is proportional to the magnitude of field strength and is given by e E or e e E where ‘αe’ is called electronic Polarizability constant It increases with increase of volume of the atom. This kind of polarization is mostly exhibited in Monatomic gases. e ____ 10-4 0F m2 He Ne Ar 0.18 0.35 1.46 It occurs only at optical frequencies (1015Hz) It is independent of temperature. Kr Xe 2.18 3.54 Expression for Electronic Polarization Consider a atom in an EF of intensity ‘E’ since the nucleus (+Ze) and electron cloud (-ze) of the atom have opposite charges and acted upon by Lorentz force (FL). Subsequently nucleus moves in the direction of field and electron cloud in opposite direction. When electron cloud and nucleus get shifted from their normal positions, an attractive force b/w them is created and the separation continuous until columbic force FC is balanced with Lorentz force FL, Finally a new equilibriums state is established. E +Ze No field fig(1) x In the presence of field fig (2) fig(2) represents displacement of nucleus and electron cloud and we assume that the –ve charge in the cloud uniformly distributed over a sphere of radius R and the spherical shape does not change for convenience. Let σ be the charge density of the sphere Ze 4 3 R 3 - Ze represent st he t ot alchargein t hesphere. T hus the- ve chargein thesphereof radius ' x' is 4 q e . .x 3 3 ze 4 3 4 . . x 3 3 . R 3 ze 3 3 x R Now Fc 1 4 0 . qe .q p x2 - - - - - (1) ze.x 3 z 2e 2 x ze - - - - - (2) 2 3 3 4 0 x R 4 0 R 1 Force experienced by displaced nucleus of Strength E is FL = Eq = ZeE -----(3) FL Fc z 2e 2 x 4 0 R 3 ZeE - - - - - (4) zex E 3 4 0 R zex zex 3 4 0 R e dipole moment E e e 4 0 R 3 Hence electronic Polaris ability is directly proportional to cube of the radius of the atom. Ionic polarization The ionic polarization occurs, when atoms form molecules and it is mainly due to a relative displacement of the atomic components of the molecule in the presence of an electric field. When a EF is applied to the molecule, the positive ions displaced by X1 to the negative side electric field and negative ions displaced by X2 to the positive side of field. The resultant dipole moment µ = q ( X1 + X2).. + + + Electric field _ anion cat ion _ _ + + _ x1 x2 + _ _ + _ + _ Restoring force constant depend upon the mass of the ion and natural frequency and is given by F eE m.w02 x or eE x m.w02 eE 1 1 x1 x2 2 m M w0 Where ‘M’ mass of anion and ‘m’ is mass of cat ion ionic e2 E 1 1 e( x1 x2 ) 2 m M w0 or ionic ionic E 2 e 1 1 2 m M w0 This polarization occurs at frequency 1013 Hz (IR). It is a slower process compared to electronic polarization. It is independent of temperature. Orientation Polarization It is also called dipolar or molecular polarization. The molecules such as H2 , N2,O2,Cl2 ,CH4,CCl4 etc., does not carry any dipole because centre of positive charge and centre of negative charge coincides. On the other hand molecules like CH3Cl, H2O,HCl, ethyl acetate ( polar molecules) carries dipoles even in the absence of electric field. How ever the net dipole moment is negligibly small since all the molecular dipoles are oriented randomly when there is no EF. In the presence of the electric field these all dipoles orient them selves in the direction of field as a result the net dipole moment becomes enormous. It occurs at a frequency 106 Hz to 1010Hz. It is slow process compare to ionic polarization. It greatly depends on temperature. Expression for orientation polarization N . o2 ri e.E Po N . o ri e N . o .E 3k T o o2 ri e 3k T el ec io n ic o ri 4 o R 3 e2 w02 1 M 1 m 3k T o2 ri This is called Langevin – Debye equation for total Polaris ability in dielectrics. Internal fields or local fields Local field or internal field in a dielectric is the space and time average of the electric field intensity acting on a particular molecule in the dielectric material. It is also known as a Microscopic field which acts at an Atom. Evaluation of internal field The internal field is electric field acting at an atom of solid or liquid dielectric subjected to an external electric field. The internal field at the atom site ‘A’ can be made up of four components E1 ,E2, E3 & E4 Which is known as internal field or Local field. i.e Ein = E1+E2+E3+E4 + + + + + + + + + ++ _ _ _ _ _ _ _ + + + + + Spherical + _ Cavity + A _ _ Spherical Cavity _ + + + + + _ _ _ _ + + + _ _ _ _ _ _ _ _ _ _ E Dielectric material Field E1: E1 is the field intensity at A due to the charge density on the plates From Field theory D E1 0 When dielectric medium is polarized due to external Electric field E, the displacement vector D is given by, D 0E P 0E P By equating these two equations… E 1 0 P ..........(1) Deviding the above equation by 0 E1 E 0 Field E2: E2 is the field intensity at A due to the charge density induced on the two sides of the dielectric due to the Polarization. E2 P 0 ...........(2) Field E3: E3 is the field due to the dipoles within the cavity which depends on the crystal structure. Here we have considered for the cubic structure so.. E3 0...........(3) + + + + + + + + + + A _ _ d _ E dA + + _ r _ _ r R p _ _ _ _ _ _ q Field E4: 1.This is due to polarized charges on the surface of the spherical cavity. dA 2 . pq.qR dA 2 .r sin .rd dA 2 .r sin d 2 Where dA is Surface area between θ & θ+dθ… 2.The total charge present on the surface area dA is… dq = ( normal component of polarization ) X ( surface area ) dq p cos dA dq 2r p cos . sin .d 2 3.The field due to this charge at A, denoted by dE4 is given by 1 dq dE4 4 0 r 2 The field in θ = 0 direction dq cos dE4 4 0 r2 1 1 2 dE4 (2r p cos . sin .d ) cos 2 4 0 r P 2 dE4 cos . sin .d 2 0 4.Thus the total field E4 due to the charges on the surface of the entire cavity is E4 dE 4 0 0 P cos2 . sin .d 2 0 P 2 0 2 cos . sin .d 0 let..x cos dx sin d P 2 0 1 2 x .dx 1 P x 3 1 P 11 ( )1 ( ) 2 0 3 2 0 3 P E4 3 0 The internal field or Lorentz field can be written as Ei E1 E2 E3 E4 p p p Ei ( E ) 0 o o 3 o p Ei E 3 o The above equation is also known as lorentz relation. So it Can be seen that local or microscopic field is larger than the macroscopic field E by an additional factor p . 3 o Classius – Mosotti relation: Consider a dielectric material having cubic structure , and assume ionic Polarizability & Orientational polarizability are zero.. i 0 0 polarization..P N P N e Ei ......where., e Ei P where., Ei E 3 0 P N e Ei P P N e ( E ) 3 0 P P N e E N e 3 0 P P N e N e E 3 0 N e P (1 ) N e E 3 0 N e E P ...................( 1) N e (1 ) 3 0 W e known t hatt hepolarizaton i vect or P 0 E ( r 1)............(2) from eq n s (1) & ( 2) N e E 0 E ( r 1) N e (1 ) 3 0 1 N e N e E 3 0 0 E ( r 1) 1 N e N e E 3 0 0 E ( r 1) 1 N e N e 3 0 0 ( r 1) 1 N e 3 (1 ) 3 0 r 1 N e 1 3 3 0 (1 ) r 1 N e 1 r ...... Classius Mosot t irelat ion 3 0 r 2 TYPES OF DIELECTRIC MATERIAL Dielectric material can be solid, liquid or gas. High vacuum can also be used as a dielectric. Solid dielectrics are most commonly use like glass, rubber, mica etc.. As a liquid dielectric material Transformer oil, cable oil, Capacitor oil, Vegetable oil etc can be used. Gaseous dielectric materials are used for both as insulators and also as a cooling agents. For example: Air, Hydrogen, nitrogen, Helium, Sulphur- dioxide, Propen, methane etc.. 1) Solid Dielectric Material: I) Mica: It is inorganic mineral material made up of silicate of aluminium with silicate of soda, potash and magnesia. It is rigid, tough and strong. It has high dielectric strength and is not affected by moisture. It is widely used in irons, hot plates and toasters. II) Glass: It is inorganic material made by the fusion of different oxides like SiO2, ZnO and MgO. It is Brittle and hard material and has good dielectric strength It is mostly used in the capacitors. Also used as dielectric tubes in radios and television. III) Asbestos: It is naturally occurring material. In general it consist of magnesium silicate. It has low dielectric strength. It is used as insulating material to prevent current flow in the outer body. It is widely used in the form of the paper, tap, cloth etc. IV) Rubber: It is organic polymer. It may be natural or synthetic. It has good electrical and thermal properties and also it has good tensile strength. It is used for the insulating materials on electrical wires. V) Ceramics: They are generally non-matalic inorganic compounds such as silicates, aluminates, oxides, carbides, borides etc. Ceramics can be classified as: clay products, refractories, and glasses. Ceramics are hard, strong and dense. They have exellent dielectric and mechanical properties. They widely used as insulators in switches, plug holders etc. They are also used as dielectric in capacitors. 2) Liquid Dielectric Material: I) Mineral Insulating Oil : These oils are obtained from crude petroleum. These have good thermal stability. They are used in Transformers as cooling and insulating material and also in Capacitors. Transformer oil, cable oil and capacitor oil belong to the category of mineral insulating oil. II) Synthetic Insulating Oil : Askarels, aroclors, sovol and savtol are a few synthetic oils that are widely used. They are very much resistant to fire hazards. Due to longer life and safety in operating condition, these oils are used as coolants and insulators in high voltage transformers in place of Transformer oil. II) Miscellaneous Insulating Oil : Vaseline, vegetable oils and silicon liquid belongs to these category. Silicon liquids has thermal stability upto 200 C and are very costly. The dielectric strength of these oils are same as mineral oils so they are also used in the H.V transformers. 3) Gaseous Dielectric Material: I) Air : It is naturally available dielectric material. Dielectric loss is practically zero. The dielectric constant of air linearly increase with increase in pressure. It is used as dielectrics in air condensers. It can be used as an insulator only in low voltage applications. II) Nitrogen : It is important gaseous dielectric material. It prevent oxidation. It is used in cables and capacitors under pressure. III) Sulphure Hexafluoride: It is formed by burning of Sulphure in fluorine atmosphere. It has superior cooling properties than air and nitrogen. It is used in the transformers, electrical switches, voltage stabilizer and X-ray apparatus. IV) Inert Gases: They are used in electronic tubes and discharge tubes as insulators. Properties of Good Dielectric Material It should have high resistivity to reduce the leakage current. It should have high dielectric strength. It should have high mechanical strength. It should have high fire resistance. It should have low thermal expansion. It should have high thermal conductivity. It should have low dielectric loss. It should have low water absorption quality. Applications of Dielectrics 1. Capacitors 2. Transformers 3. Polymeric film 4. Electrolytic 5. Power and Distribution transformers 6. Other applications