Chapter 4 Other Techniques: Microscopy, Spectroscopy, Thermal
advertisement

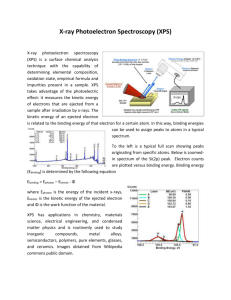
Chapter 4 Other Techniques: Microscopy, Spectroscopy, Thermal Analysis Microscopic techniques • Optical microscopy - polarizing microscope - reflected light microscope • Electron microscopy - scanning electron microscopy (SEM) - transmission electron microscopy (TEM) - high resolution electron microscopy (HREM) EDS: Energy Dispersive Spectroscopy Applications • Optical microscopy - phase identification, purity, and homogeneity - crystal defects : grain boundaries and dislocation - refractive index determination • Electron microscopy - particle size and shape, texture, surface detail - crystal defects - precipitation and phase transitions - chemical analysis - structure determination SEM scanning electron microscopy Photos of SEM EDS Energy Dispersive Spectroscopy An attachment of EM Transmission Electron Microscopy TEM Wavelength of electrons = h(2meV)-1/2 At 90 kV accelerating voltage, ~ 0.04 Å Consequently, the Bragg angles for diffraction are small and the diffracted beams are concentrated into a narrow cone centered on the undiffracted beam. Basic components of a TEM HREM of an intergrowth tungsten bronze, Rb0.1WO3 Scanning tunneling microscope (STM) The STM can obtain images of conductive surfaces at an atomic scale of 0.2 nm, and also can be used to manipulate individual atoms, trigger chemical reactions, or reversibly produce ions by removing or adding individual electron from atoms or molecules. Atomic force microscope (AFM) Field Emission SEM (FESEM) Traditional SEM: Thermionic Emitters use electrical current to heat up a filament FESEM: A Field Emission Gun (FEG); also called a cold cathode field emitter, does not heat the filament. The emission is reached by placing the filament in a huge electrical potential gradient. FESEM uses Field Emission Gun producing a cleaner image, less electrostatic distortions and spatial resolution < 2nm. Spectroscopic techniques Vibrational spectroscopy : IR and Raman • IR Raman Visible and ultraviolet spectroscopy UV/visible Nuclear magnetic resonance (NMR) spectroscopy Magic Angle Spinning NMR (MAS-NMR) If a solid-state sample is allowed to spin at an angle of θ=54.7° to a strong external magnetic field, dipolar coupling (D) will be zero. Example 1 Example 2 Electron spin resonance (ESR) spectroscopy : detect unpaired electrons X-ray spectroscopy : XRF, AEFS, EXAFS X-ray fluorescence (XRF) -coordination number -bond distance -oxidation state X-ray absorption techniques • Absorption edge fine structure (AEFS) or X-ray absorption near edge structure (XANES) Information can be obtained - oxidation state, site symmetry, surrounding ligands, the nature of the bonding • Extended X-ray absorption fine structure (EXAFS) Information can be obtained - bonding distance, coordination number Extended X-Ray Absorption Fine Structure This introduction to the theory of EXAFS is divided into basic, relatively simple and complicated parts. EXAFS spectra are a plot of the value of the absorption coefficient of a material against energy over a 500 - 1000 eV range (including an absorption edge near the start of the spectrum). Through careful analysis of the oscillating part of the spectrum after the edge, information relating to the coordination environment of a central excited atom can be obtained. The theory as to what information is contained in the oscillations is described here. EXAFS EXAFS XANES AEFS (or XANES) Electron spectroscopies • • • • • ESCA XPS UPS AES EELS Origins of ESCA and Auger spectra Electron Spectroscopy for Chemical Analysis: XPS, UPS Auger electrons are secondary electrons Core, valence and virtual levels X-ray photoelectron spectroscopy XPS is a surface chemical analysis technique XPS is used to measure: 1) elemental composition of the surface (1–10 nm usually) 2) empirical formula of pure materials 3) elements that contaminate a surface 4) chemical or electronic state of each element in the surface 5) uniformity of elemental composition across the top of the surface (line profiling or mapping) 6) uniformity of elemental composition as a function of ion beam etching (depth profiling) XPS and UPS • XPS: core-level photoelectron spectroscopy • UPS: valence-level photoelectron spectroscopy hv = Ek + ef + Eb Ek = kinetic energy of escaped electrons ef = work function (energy from Fermi level to continuous states) Eb = binding energy Ek is measured experimentally Eb contains information of electronic structure Schematic representation of hv = Ek + ef + Eb Ek ef hv Eb XPS Resolution of XPS and UPS • XPS conventionally has lower resolution (0.2 ~1.2 eV). Cannot see vibration (< 0.5 eV or 4000 cm-1) • UPS has better resolution ( < 0.01 eV). Can see vibration frequency. • For UPS, hv = Ek + ef + Eb +DEvib Synchrotron-based light source can enhance resolution Different oxidation states determined by XPS Example of "High Energy Resolution XPS Spectrum" also called High Res spectrum. This is used to decide what chemical states exist for the element being analyzed. In this example the Si (2p) signal reveals pure Silicon at 99.69 eV, a Si2O3 species at 102.72 eV and a small SiO2 peak at 103.67 eV. The amount of Si2O at 100.64 eV is very small. XPS and AES XPS spectra of Na2S2O3 and Na2SO4 XPS spectrum of KCr3O8 XPS spectrum of NaWO3 Band Structures can be seen by XPS The relative amount of electrons filled in a band can be seen. XPS of Co and its oxide Binding Energy Table Band Structures can be seen by XPS An example of UPS Franck-Condon Principle and Changes of Vibration Frequencies UPS of O2 antibonding bonding Thermal analysis • Thermogravimetry (TGA) • Differential thermal analysis (DTA) • Differential scanning calorimetry (DSC) • Thermomechanical analysis (TMA) Setup of Thermogravimetric Analysis Thermogravimetric Analysis (TGA) measures weight changes in a material as a function of temperature (or time) under a controlled atmosphere. TGA curve Heating rate dependence of TGA curve CuSO4.5H2O heated in air in a Pt crucible TGA of CaCO3 Possibility of Mixtures The DTA method Differential Thermal Analysis (DTA) measures the difference in temperature between a sample and a thermally inert reference as the temperature is raised. The plot of this differential provides information on exothermic and endothermic reactions taking place in the sample. Variation of peak temperature of kolin with rate of temperature increase TGA and DTA curves of kaolin minerals Application of DTA 1 : melting/solidification Application of DTA 2 : melting behavior of crystal Application of DTA 3 : phase diagram determination Application of TGA : stepwise decomposition of Ca(COO)2·H2O Conversion of TGA to DTG Differential Scanning Calorimetry (DSC) Differential Scanning Calorimetry (DSC) is a thermal analysis technique which is used to measure the temperatures and heat flows associated with transitions in materials as a function of time and temperature. Thermomechanical analysis (TMA) Thermomechanical analysis (TMA) is used to determine the deformation of a sample (changes in length or thickness) as a function of temperature. Linear Variable Displacement Transducer Mössbauer spectroscopy • Oxidation state, coordination numbers, bond character Mössbauer (MB) Spectroscopy Nuclear transition: absorption of g-rays by sample. The condition for absorption depend on the electron density about the nucleus and the number of peaks obtained is related to the symmetry of the compound. The energy of emitted Eg = Er + D – R Er : Eexcited state – Eground state of the source nucleus D: the Doppler shift due to the transitional motion of the nucleus R : the recoil energy of the nucleus The energy of the g-ray absorbed Eg = Er + D + R Distribution of energy of emitted and absorbed g-rays Emitted Eg = Er + D - R = Er + D + R = Eg absorbed The main cause for non matching of g-rays energies is the recoil energy. R 10-1 eV for a gaseous molecule Doppler effect D 2 104 cm sec-1 R can be reduced by increasing mass by placing the nucleus of the sample and source in a solid. PA = mvR = -Eg/c R = mvR2/2 = PA2/2m = Eg2/2mc2 P = momentum 2 R= Eg 2mc 2 Three main types of interaction of the nuclei with the chemical environment: 1. Resonance line shifts form changes in electron environment 2. Quardrupole interactions 3. Magnetic interactions isomer shifts, center shifts, chemical shifts MB of Fe3+FeIII(CN)6 Fe3+: weak field 0.53 mm sec-1 FeIII: strong field 0.03 mm sec-1 The isomer shift results from the electrostatic interaction of the charge distribution in the nucleus with the electron density that has a finite probability of exiting at the nucleus. Only s electrons have a finite probability of overlapping in the nuclear density. p, d and other electron densities screening effect Dr 2 IS D s (0) r Dr = re rg Dr/r is positive and any factor which increases in the total s electron density increases the isomer shift (IS, CS) 1. Increase in the covalent bonds 2. Decrease the p or d-populations. Increase in oxidation state of a transition metal. 3. Decrease in coordination number Tetrahedral (sp3) more s character higher IS Octahedral (d2sp3) Dr/r is negative for Fe Fe2+ (d6) has an appreciably larger center shift than Fe3+ (d5) Isomer Shift for High Spin Iron Compounds +1 Oxidation state ~+2.2 Isomer shift +2 ~+1.4 +3 +4 ~+0.7 ~+0.2 +6 ~-0.6 Quadrupole interactions Quadrupole splitting arises from the presence of an electron filed gradient (EFG) at the nucleus. The magnitude of the QS represents the asymmetry of the electron cloud around the nucleus. QS IS IS QS 57Fe, 119Sn 197Au The I = 3/2 state is split into two sublevels by the presence of an electron-field gradient. The magnitude of the QS is one-half of the quadrupole coupling constant (QCC) for the I = 3/2 state. Strong field case t2g6 Low spin case Fe(II) NO quadrupole weak field case t2g4eg2 high spin case Fe(II) large quadrupole In octahedral geometry only d3, d8, d10 , high spin d5 and low spin d6 configurations make no contribution to the QS. Mössbauer of KFeS2