Influence of Acceptor Structure on Barriers to Charge
advertisement
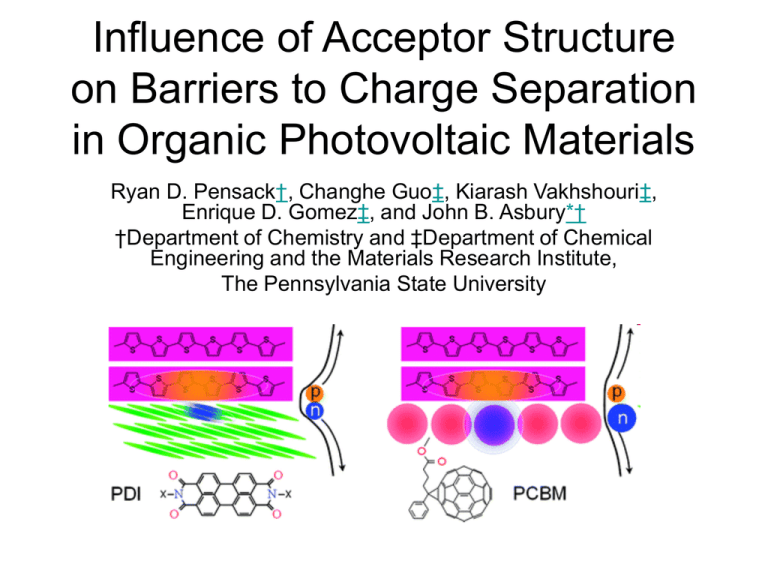
Influence of Acceptor Structure on Barriers to Charge Separation in Organic Photovoltaic Materials Ryan D. Pensack†, Changhe Guo‡, Kiarash Vakhshouri‡, Enrique D. Gomez‡, and John B. Asbury*† †Department of Chemistry and ‡Department of Chemical Engineering and the Materials Research Institute, The Pennsylvania State University Background • Charge Transfer States – Bound electron/hole pair at the interface of the donor and acceptor – CT state can be formed through electron transfer from donor to acceptor or hole transfer from acceptor to donor – The binding energy of this CT state is 0.0 -0.5 eV – Additional studies have shown that excess energy is not require to dissociate this CT state because of a minimal temperature dependence. • Current work – Identify the factors that may influence charge separation in OPV materials using ultrafast vibrational spectroscopy – P3HT as the Donor – PC61BM and BTBP-PDI as acceptors – Measure the rates of CT state dissociation by using an approach based on solvatochromism assisted vibrational spectroscopy (SAVS) Experimental • SAVS (TRIR) – Films were prepared from chlorobenzene with 300-500 nm thickness – Vis-IR experiment designed to only excite the donor – Probe with IR – 1 ps resolution – 50 mW/cm2 power density • X-Ray diffraction – Examine the crystalline nature of the films Results: PDI • P3HT:BTBP-PDI ultrafast C=O dynamics 300K – Broad feature contains positive polarons in P3HT, negative polarons in PDI and ground state bleach – Fit each of the three components and extract a center frequency for the bleach which is traced by the dotted line – Shift to lower energy is indicative of CT state dissociation • Temperature Dependence on center frequency of the bleach – Cooler temperatures slow charge transfer dynamics Results: PCBM • Broad feature contains polarons and bleach 325 K – Extract bleach from 3 component fit – Bleach shift is must less sensitive than in PDI – Slower CT state dissociation (ns) • Temperature Dependence – CT state dissociation is not sensitive to temperature – Nanosecond timescale at all temperatures Asymptotic Behavior of Shifts • G(t) represents experimental frequency shifts of the kinetic trace at a particular temperature • G(∞) represents the asymptotic shifts at long times. – PDI: 1709 to 1705 cm-1 – PCBM: 1739 to 1740 cm-1 – Consistent with previous experiments with different donors – Likely the result of a thermal contraction of the donor – Origin of the greater sensitivity in PDI is currently under investigation Film Morphology • Crystalline P3HT in all films • Amorphous PCBM and BTBP:PDI • Crystalline C8:PDI Reaction Barriers • Plot reaction rate vs. 1/T • PDI shows significant dependence but with nonarrhenius behavior – Extract a binding energy of 0.1eV • PCBM shows no dependence indicating a barrierless pathway BTBP:PDI Blend Barrier • Conclude the charge separation is activated • Time scale for CT state dissociation ranges from 1ps to 10ps depending on the temperature • Excess energy model: Ground state, photosynthetic reaction centers – At lower temperature the dynamics are sufficiently slowed such that vibrational energy redistribution dissipates the energy before the CT state dissociates. Thus thermal energy is required. • Competing Model – Authors believe the system is activated at all temperatures – Non-Arrhenius behavior arises from the dynamics being to fast to be clearly resolved. – The vibrational energy redistribution is much quicker in the excited state vs the ground state – Needs a faster experiment to make a confident assignment PCBM Barrierless Separation • Nanosecond timescale indicates excess energy does not play a role since it would be dissipated on that timescale • Recent OPV device studies on the same blend do not show temperature dependence of the VOC • Literature Insights – Electronic wave function of the PCBM is delocalized and the reorganization energy is less because of this delocalization – Molecular species show a larger coulombic attraction because of smaller spatial confinement of the charges – In PCBM, the wavefunctions are so delocalized that Coulombic barriers and reorganization energies are reduced below the energetic disorder of the system Structure Influence on the Barrier • PCBM – 1 nm3 volume and 60 carbon atoms to distribute the charge – Diffuse electron even if the charge is only on one fullerene – Non-directional because of the spherical symmetry of PCBM • BTBP:PDI – 20 atoms and 1/3 the volume of PCBM – Requires multiple PDI molecules to distribute the charge of the same spatial volume as compared with PCBM – The faster (1ps) charge separation PDI molecules; however, the lack of a crystalline phase prevents delocalization – The activation barrier combined with the lack of evidence of a crystalline phase suggests that electron localization causes a greater coulombic binding energy of the CT state – Exploring C8:PDI blend because of its crystallinity Conclusion • Examined the dynamics of OPV materials by monitoring the C=O vibration of photoreduced electron acceptors using TRIR • The PCBM blend shows a barrierless dissociation of the CT state • The BTBP:PDI requires energy to activate the CT state dissociation as indicated by the temperature dependence • X-Ray measurements show the PCBM does not require an ordered phase to achieve sufficient charge delocalization • The PDI acceptors may require a greater degree of crystallinity to achieve sufficient delocalization • Proposed that C8:PDI blend would show less barriers to charge separation