งานนำเสนอ PowerPoint
advertisement
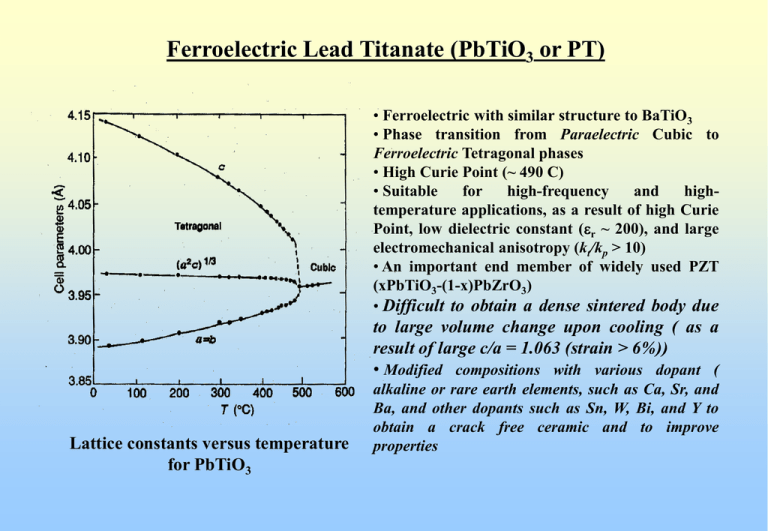
Ferroelectric Lead Titanate (PbTiO3 or PT) • Ferroelectric with similar structure to BaTiO3 • Phase transition from Paraelectric Cubic to Ferroelectric Tetragonal phases • High Curie Point (~ 490 C) • Suitable for high-frequency and hightemperature applications, as a result of high Curie Point, low dielectric constant (er ~ 200), and large electromechanical anisotropy (kt/kp > 10) • An important end member of widely used PZT (xPbTiO3-(1-x)PbZrO3) • Difficult to obtain a dense sintered body due to large volume change upon cooling ( as a result of large c/a = 1.063 (strain > 6%)) • Modified compositions with various dopant ( Lattice constants versus temperature for PbTiO3 alkaline or rare earth elements, such as Ca, Sr, and Ba, and other dopants such as Sn, W, Bi, and Y to obtain a crack free ceramic and to improve properties (Ferroelectric?) Lead Zirconate (PbZrO3 or PZ) Lattice constants versus temperature (in pseudo-tetragonal) for PbZrO3 Dielectric constant versus temperature for PbZrO3 ceramic Above 230 C, the structure is cubic-perovskite (similar to BaTiO3) Dielectric constant shows “anomaly” at 230 C (reaches high peak) Above 230 C, dielectric constant follows Curie-Weiss relation Ferroelectric Material ? No ferroelectric hysteresis below “transition temperature” 230 C Volume contraction upon cooling (c/a < 1) Orthorhombic Structure Anti-Ferroelectric Material Antiferroelectricity Antiferroelectricity Anti-ferroelectric materials Non-polar, Nonferroelectric materials that revert to a ferroelectric state when subjected to sufficiently high electric field, causing a “double-loop hysteresis” Lead Zirconate Titanate (Pb (Zrx Ti1-x)O3 or PZT) System PZT (xPbZrO3 – (1-x)PbTiO3) Binary Solid Solution PbZrO3 (antiferroelectric matrial with orthorhombic structure) and PbTiO3 (ferroelectric material with tetragonal perovskite structure) Perovskite Structure (ABO3) with Ti4+ and Zr4+ ions “randomly” occupying the B-sites Important Transducer Material (Replacing BaTiO3) • Higher electromechanical coupling coefficient than BaTiO3 • Higher Tc results in higher operating and fabricating temperatures • Easily poled • Wider range of dielectric constants • relatively easy to sinter at lower temperature than BaTiO3 • form solid-solution compositions with several additives which results in a wide range of tailored properties Lead Zirconate Titanate (Pb (Zrx Ti1-x)O3 or PZT) System PZT Solid Solution Phase Diagram Zr/Ti ratio 52/48 MPB Lead Zirconate Titanate (Pb (Zrx Ti1-x)O3 or PZT) System PZT Solid Solution Phase Diagram Zr/Ti ratio 52/48 MPB showing structure changes Lead Zirconate Titanate (Pb (Zrx Ti1-x)O3 or PZT) System Abrupt changes in lattice constants at room temperature for PZT system lead to anomalous behaviors in dielectric and piezoelectric properties Variation of polarization with PZ contents indicates that the highest polarization is in the rhombohedral structure that does not have highest er and d Lead Zirconate Titanate (Pb (Zrx Ti1-x)O3 or PZT) System Composition dependence of dielectric constant (K) and electromechanical planar coupling coefficient (kp) in PZT system This shows enhanced dielectric and electromechanical properties at the MPB Increased interest in PZT materials with MPB-compositions for applications Lead Zirconate Titanate (Pb (Zrx Ti1-x)O3 or PZT) System Electromechanical coupling coefficients Piezoelectric d constants Variation of room temperature piezoelectric properties with PZT compositions Note: highest values on tetragonal side of the composition Lead Zirconate Titanate (Pb (Zrx Ti1-x)O3 or PZT) System Piezoelectric g strain coefficients Dielectric constants Variation of room temperature piezoelectric properties with PZT compositions Note: highest dielectric constants on tetragonal side of the composition BUT high piezoelectric g strain coefficients into rhombohedral side Lead Zirconate Titanate (Pb (Zrx Ti1-x)O3 or PZT) System Possible domain states Value of the mixed phase region at the MPB in poling of PZT vs other perovskite ferroelectrics Lead Zirconate Titanate (Pb (Zrx Ti1-x)O3 or PZT) System Advantages of PZT Solid-Solution System • Above Curie Point (or Curie Temperature), the symmetry is cubic with perovskite structure • High Tc across the diagram leads to more stable ferroelectric states over wide temperature ranges • There is a two-phase region near the Morphotropic Phase Boundary (MPB) (52/48 Zr/Ti composition) separating rhombohedral (with 8 domain states) and tetragonal (with 6 domain states) phases • In the two-phase region, the poling may draw upon 14 orientation states leading to exceptional polability • Near vertical MPB results in property enhancement over wider temperature range for chosen compositions near the MPB Compositions and Modifications of PZT System 1. Effects of composition and grain size on properties MPB compositions (Zr/Ti = 52/48) Maximum dielectric and piezoelectric properties Selection of Zr/Ti can be used to tailor specific properties High kp and er are desired Near MPB compositions OR High Qm and low er are desired Compositions away from MPB Grain Size (composition and processing) Fine-Grain ~ 1 mm or less Coase-Grain ~ 6-7 mm Some oxides are grain growth inhibitor (i.e. Fe2O3) Some oxides are grain growth promoter (i.e. CeO2) Dielectric and piezoelectric properties are grain-size dependent Compositions and Modifications of PZT System Dependence of dielectric and piezoelectric properties on average grain size in the ceramic Pb(Zr0.51Ti0.49)O3 + 0.1 wt% MnO2 at a constant density of 7.70-7.85 g/cm3 Piezoelectric properties increase linearly with increasing grain size Compositions and Modifications of PZT System 2. Modification by element substitution Element substitution cations in perovskite lattice (Pb2+, Ti4+, and Zr4+) are replaced partially by other cations with the same chemical valence and similar ionic radii and solid solution is formed Pb2+ substituted by alkali-earth metals, Mg2+, Ca2+, Sr2+, and Ba2+ PZT replaced partially by Ca2+or Sr2+ Tc BUT kp, e33 , and d31 Shift of MPB towards the Zr-rich side Density due to fluxing effect of Ca or Sr ions Ti4+ and Zr4+ substituted by Sn4+ and Hf4+ , respectively Ti4+ replaced partially by Sn4+ c/a ratio decreases with increasing Sn4+ content Tc and stability of kp and e33 Compositions and Modifications of PZT System 3. Influences of low level “off-valent” additives (0-5 mol%) on dielectric and piezoelectric properties Two main groups of additives: 1. electron acceptors (charge on the replacing cation is smaller) (A-Site:K+, Rb+ ; B-Site: Co3+, Fe3+, Sc3+, Ga3+, Cr3+, Mn3+, Mn2+, Mg2+, Cu2+) (Oxygen Vacancies) Reduce both dielectric and piezoelectric responses Increase highly asymmetric hysteresis and larger coercivity Much larger mechanical Q “Hard PZT” 2. electron donors (charge on the replacing cation is larger) (A-Site: La3+, Bi3+, Nd3+; B-Site: Nb5+, Ta5+, Sb5+) (A-Site Vacancies) Enhance both dielectric and piezoelectric responses at room temp Under high field, symmetric unbiased square hysteresis loops low electrical coercivity “Soft PZT” Lead Zirconate Titanate (Pb (Zrx Ti1-x)O3 or PZT) System Dielectric constant vs temperature of various types PZT materials Modified PZT System 3.1 Hard Doping: Hard PZT (A-Site:K+, Rb+ ; B-Site: Co3+, Fe3+, Sc3+, Ga3+, Cr3+, Mn3+, Mn2+, Mg2+, Cu2+) Oxygen Vacancies in either A-sites or B-sites or Both (Electroneutrality) Two replaced by two ions OR Two Zr4+ (or Ti4+) replaced by two Fe3+ ions Space charges inhibit domain motion and Insoluble doped ions inhibit grain growth Pb2+ K+ Increased Qm and Ec, Decreased loss tangent, and Lowered dielectric and piezoelectric activities “Hard PZT” Rugged Applications (High Temperature and High Driving Loads) Modified PZT System 3.2 Soft Doping: Soft PZT (A-Site: La3+, Bi3+, Nd3+; B-Site: Nb5+, Ta5+, Sb5+) A-Site Vacancies (Electroneutrality) Two Pb2+ replaced by two La3+ ions OR Two Zr4+ (or Ti4+) replaced by two Nb5+ ions Easier transfer of atoms leads to increased domain motion at lower electric filed ( Ec) Internal stress relieve more easily Increased domain wall mobility Lowered Qm and Ec, Increased loss tangent, and Increased dielectric and piezoelectric activities “Soft PZT” Applications required higher piezoelectric activities (Sensors, Actuators, and Transducers) Modified PZT System “Hard PZT” Materials Curie temperature above 300 C NOT easily poled or depoled except at high temperature Small piezoelectric d constants Good linearity and low hysteresis High mechanical Q values Withstand high loads and voltages “Soft PZT” Materials Lower Curie temperature Readily poled or depoled at room temperature with high field Large piezoelectric d constants Poor linearity and highly hysteretic Large dielectric constants and dissipation factors Limited uses at high field and high frequency Modified PZT System 3.3 Other Doping Ions (Ce, Cr, U, Ag, Ir, Rh, Ni, Mn, Nb, Al) Ce-doped PZT high r, Qm, Qe, e, Ec, kp Cr-doped PZT high Qm, tan d, with lower kp U-doped PZT high Qm, r , and tan d Ag, Ir, or Rh-doped PZT high Qm, kp , and lower e Complexed doping (with two or more metal elements) Better than single ion doping (enhances both Qm and kp) Compound Dopings (BiFeO3, AgSbO3 or Ca2Fe2O5) Reducing dielectric loss at high field Examples of Practical Modified PZT System 1. Materials for ceramic filters: 1.1 Range 30-150 MHz (modified PT) 0.99[0.96PbTiO3 + 0.04 La2/3TiO3] + 0.01MnO2 1.2 Range 10-20 MHz Pb1.03[(Nb2O6)0.07(CrO2)0.03(Zr0.52Ti0.48O3)0.90] + 0.5 wt%MnO2 + 1 wt% La2O3 1.3 Range 1-10 MHz Pb0.95Sr0.05Mg0.03(Zr0.52Ti0.48)O3 + 0.5 wt%CeO2 + 0.225 wt% MnO2 2. Materials for underwater ultrasonic transducers: Pb0.95Sr0.05 (Zr0.54Ti0.46)O3 + 0.9 wt%La2O3 + 0.9 wt% Nb2O5 3. Materials for high-voltage generator: [Pb(Nb1/2Sb1/2)O3]0.05 [PbTiO3]0.41 [PbZrO3]0.54 + 0.2 mol%Nb2O5 + 0.2 mol%Y2O3 + 0.1 wt% MnO2 4. Materials for electro-acoustic applications: [Pb(Ni1/3Nb2/3)O3]0.50 [PbTiO3]0.355 [PbZrO3]0.145 Lead Zirconate Titanate (Pb (Zrx Ti1-x)O3 or PZT) System Examples of Commercially Available PZT from APC Lead Zirconate Titanate (Pb (Zrx Ti1-x)O3 or PZT) System Examples of Commercially Available PZT from PKI Navy Type I (PKI-402 and PKI-406) High power and low losses for driver applications (ultrosonic cleaners, fish finders, medical applications, and sonars) Navy Type II (PKI-502) High electromechanical activity and high dielectric constant for receiver applications (hydrophones, phono pickups, sound detectors, accelerometers, delay lines, flow detectors, and flow meters) Navy Type III (PKI-802 and PKI-804) High Q and low losses under extreme driving conditions for medical applications Navy Type V (PKI-532) High sensitivity, high dielectric constant and low impedance for sensor applications Navy Type VI (PKI-552 and PKI-556) High dielectric constant and large displacement for sensor applications PKI-556 is modified to give higher g33, higher k33, and lower loss factor La-Doped Lead Zirconate Titanate (PLZT) System (Pb1-xLax)(Zr1-yTiy)1-x/4VB0.25xO3 and (Pb1-xLax)1-x/2(Zr1-yTiy)VAx/2O3 La3+ into A-sites with B-site-vacancies created and Vacancies created on A-sites (Charge Balance with combination of both A and B-sites vacancies) Perovskite Structure (ABO3) similar to BaTiO3 and PZT Important (First) Transparent Ceramic (Replacing Single Crystals) (Prepared by Chemical Co-Precipitation and Hot-Press Sintering in Oxygen Atmosphere) • Increased squareness of the hysteresis loop • Decreased coercive field and increased dielectric constant • Maximum coupling coefficients and increased mechanical compliance • Enhanced optical transparency As a result of high solubility of 3+ La in the oxygen octahedral structure Series of single-phase solid-solution compositions Less unit-cell distortion and reduced optical anisotropy Uniform grain-growth and densification leads to single-phase, pore-free microstructure La-Doped Lead Zirconate Titanate (PLZT) System (Pb1-xLax)(Zr1-yTiy)1-x/4VB0.25xO3 ( x ~ 2-30 at%) Notation x / y / (1-y) 8/65/35 Pb0.92La0.08(Zr0.65Ti0.35)0.98O3 Majority of Research PLZT 6-9/65/35 In the phase diagram Phase Diagram of the PZT and PLZT Solid-Solution Systems 1) La solubility depends on PZ/PT 2) Excess La results in reduced transparency due to mixed phases of PLZT, La2Zr2O7, and La2Ti2O7 3) La reduces Tc 4) MPB compositions have enhanced properties La-Doped Lead Zirconate Titanate (PLZT) System MPB Ferroelectric tetragonal and rhombohedral phases Pyroelectric Applications Orthorhombic Anti-ferroelectric phases MPB Compositions for transducer applications Cubic paraelectric phases Diffused metastable relaxor (electrically inducible to ferroelectric) for quadratic strain and electro-optics Phase Diagram of the PZT and PLZT Solid-Solution Systems La-Doped Lead Zirconate Titanate (PLZT) System Room Temperature Phase Diagram of PLZT with representative hysteresis loops at various compositions La-Doped Lead Zirconate Titanate (PLZT) System PbZrO3 Complex relationship between n and E Mole% PbZrO3 PbTiO3 n ~ E n ~ E2 Room Temperature Phase Diagram of PLZT showing different electro-optic characteristics at various compositions La-Doped Lead Zirconate Titanate (PLZT) System Electro-optic applications of PLZT ceramics depends on the composition 8/40/60 linear region In the tetragonal ferroelectric (FT) region high EC hysteresis loops Linear electro-optic behavior for E < EC (linear electro-optic modulators, and optical switches) In the rhombohedral ferroelectric (FR) Low EC hysteresis loops Optical memory applications (light valves, optical-storage-display devices) 8/65/35 memory region 9/65/35 quadratic region PLZT ceramic compositions with the relaxor ferroelectric behavior are characterized by a slim hysteresis loop Large quadratic electro-optic effects for making flash protection goggles La-Doped Lead Zirconate Titanate (PLZT) System Examples of Applications of PLZT Ceramics Flash Goggles : nuclear and arc radiations Color Filter : optical shutter Display : reflective display similar to LCD Image Storage : strain-induced birefringence Thin-Film Optical Switch Photostriction