Topology_in_the_solid_state_sciences
advertisement
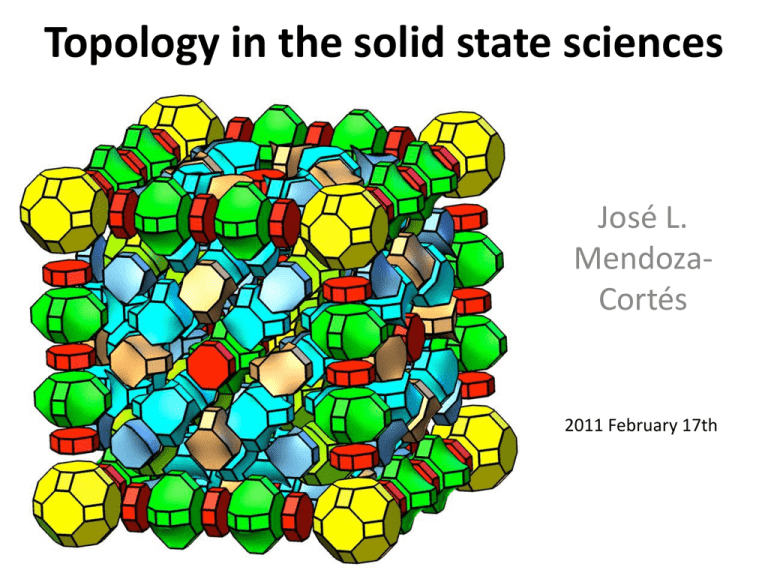
Topology in the solid state sciences
José L.
MendozaCortés
2011 February 17th
Why is it important? What can we learn?
Physics
Materials
Science
Chemistry
Biology
What do they mean by Topology?
Main Questions
• Fundamental question:
Given an spectra (e.g.
sound), can you tell the
shape of the source (e.g. the
instrument shape)
• In other words: Is it possible
that two molecules or solids
can have the same
properties, given the only
difference is their topology?
Topology is concerned with
spatial properties that are
preserved under continuous
deformations of objects.
Familiarity
Voronoi-Dirichlet polyhedron
Wigner-Seitz cell
First Brillouin zone
All are example of Voronoi-Dirichlet
polyhedron but applied to an specific
field
Everything we are going to cover today it comes to this!
And this:
Zeolites
From real stuff to abstract stuff
node
rod
Different topologies could be obtained
on varying the coordination geometry of
the nodes...
From real stuff to abstract stuff
honeycomb layer
Lets see abstract stuff
“Topological”
Entanglement
“Euclidean”
Entanglement
Borromean links
Lets see abstract stuff
Models: Lattice
hxl/Shubnikov plane net (3,6)
Atom coordinates
C1 0.00000 0.00000 0.00000
Space Group: P6/mmm
Cell Dimensions
a=1.0000 b=1.0000 c=10.0000
Crystallographic, not crystallochemical model
Models: Net
Inherently crystallochemical, but no
geometrical properties are analyzed
Models: Labeled quotient graph
Chung, S.J., Hahn, Th. & Klee, W.E. (1984). Acta Cryst. A40, 42-50.
010
2
0 1 0
100
2
1 00
001
2
00 1
010
2
0 1 0
001
2
001 00 1
2
00 1
010
2
0 1 0
100
2
1 00
a
Wrapping NaCl 3D graph
100
2
1 00
001
2
00 1
100
2
1
00
010
2
0 1 0
b
NaCl labeled quotient graph
Models: Embedded net
Diamond (dia) net in the most symmetrical embedding
Models: Polyhedral subdivision
Voronoi-Dirichlet polyhedron and partition: bcu net
Kd=0.5
Models: Polyhedral subdivision
Tilings: dia and bcu nets
dia
‘Normal’ crystal chemistry -> ‘dual’ crystal chemistry
bcu
Abstract stuff
4
3-connected
graph means
that the three
vertex are
connected with
other three
vertex
(therefore they
have three
edges)
Where can we apply this?
Hsieh, D. et al. A tunable topological insulator in the spin helical Dirac transport regime.
Nature 460, 1101–1105 (2009).
Where can we apply this?
world records of
Interpenetration
2002
10-fold dia MOF
Ag(dodecandinitrile)2
11-fold dia H-bond
[C(ROH)4][Bzq]2
Class Ia
...
18-fold srs H-bond
(trimesic acid)2(bpetha)3
Class IIIb
dia 12f
2008
12 interpenetrating nets
TIV: [0,1,0] (13.71A)
NISE: 2(1)[0,0,1]
Zt=6; Zn=2
Class IIIa Z=12[6*2]
#########################################
12;RefCode:SOBTUY:C40 H42 Cd2 N12 O21 Pd1
Author(s): Abrahams B.F.,Hoskins B.F.,Robson R.
Journal: J.AM.CHEM.SOC. Year: 1991 Volume: 113 Number: Pages: 3606
#########################################
-------------------Atom Pd1 links with
R(A-A)
Pd 1
0.5000
-0.5000
1.0000
( 0-1 1)
19.905A
Pd 1
-1.0000
0.0000
-1.5000
(-1 0-2)
17.126A
Pd 1
1.0000
0.0000
1.5000
( 1 0 1)
17.126A
Pd 1
-0.5000
0.5000
-1.0000
(-1 0-1)
19.905A
------------------------Structure consists of 3D framework with Pd
(SINGLE NET)
Coordination sequences
---------------------Pd1: 1 2 3
4
5
6
7
8
9
10
Num
4 12 30 58 94 138 190 250 318 394
Cum
5 17 47 105 199 337 527 777 1095 1489
---------------------Vertex symbols for selected sublattice
------------------- ------------------Pd1 Point/Schlafli symbol:{6^5;8}
With circuits:[6.6.6.6.6(2).8(2)]
With rings:
[6.6.6.6.6(2).*]
-------------------------------------Total Point/Schlafli symbol: {6^5;8}
TOPOS OUTPUT
4-c net; uninodal net
Classification of the topological type: cds/CdSO4 {6^5;8} - VS [6.6.6.6.6(2).*]
O’Keffe & Delgado-Friedrichs
3dt
2002
SyStRe
2003
Symmetry Structure Realization
one can determine without ambiguity
whether two nets are isomorphic or
not
SyStRe
3dt
3D Tiling
Thanks to:
Delgado-Friedrich, O’Keeffe, Hyde,
Blatov, Proserpio.
Suplementary slides
Suplementary slides
Self-entanglement
POLYCATENATION
INTERPENETRATION
a) 0D 1D
b) 0D 1D
c) 1D 2D
e) 2D 3D
d) 1D 3D
f) 2D 3D
increase of
dimensionality
dimensionality
unchanged
..\libro_braga\figure\asufig.jpg
Borromean
entanglements
Polycatenation
“Topological”
self-catenation
Interpenetration
“Euclidean”
Polythreading
A new complexity of the solid state
Data: Electronic crystallographic databases
CSD ~430000 entries
ICSD ~100000 entries
CrystMet ~100000 entries
PDB ~50000 entries
Data: Electronic crystallochemical databases
RCSR 1620 entries; http://rcsr.anu.edu.au
TTD Collection 66833 entries;
http://www.topos.ssu.samara.ru
TTO Collection 3617 entries;
http://www.topos.ssu.samara.ru
Atlas of Zeolite Frameworks, 179 entries;
http://www.iza-structure.org/databases/
Data: Electronic databases of hypothetical nets
EPINET 14532 entries; http://epinet.anu.edu.au/
Atlas of Prospective Zeolite Frameworks
2543772 entries;
http://www.hypotheticalzeolites.net/
History of crystallochemical analysis
Mathematical fundamentals
J. Hessel, 1830 – 32 geometric crystal classes
O. Bravais, 1848 – 14 three-periodic lattices
E. Fedorov and A. Shönflies, 1890 – 230 space groups
History of crystallochemical analysis
Microscopic observations
M. Laue, 1912 – diffraction of X-rays in crystals
W.G. Bragg and W.L. Bragg, 1913 – first structure determinations
History of crystallochemical analysis
Experimental technique and methods of X-ray analysis
1920s – 1960s
Photomethods and technique
First printed manuals on crystal structures
First really crystallochemical laws –
(L. Pauling, V. Goldschmidt, A. Kitaigorodskii)
A.F. Wells, 1954 – graph representation
History of crystallochemical analysis
Time of automated diffractometers
1960s – present time
Rapid accumulation of experimental data
Now the number of determined crystal structures exceeds
600,000 and grows faster and faster
Algorithms: building adjacency matrix
Method of
intersecting spheres
For inorganic compounds
Method of spherical
sectors
For organic, inorganic and metal-organic
compounds
Distances
For all types of compounds, using atomic
radii and Voronoi polyhedra
Solid Angles
For artificial nets, intermetallides, noble
gases, using Voronoi polyhedra
Van der Waals
Specific
Valence
Valence
Valence
Algorithms: building adjacency matrix
Solid angle of a VDP face is
proportional to the bond
strength
Topological insulators
an extremely short explanation y Jose L. Mendoza-Cortes
•It is an insulator (or a semiconductor) at bulk
•At the surface, new states appears (The so called surface states)
•These new states suffer from spin-orbit coupling
•These surface states determines if they are topological insulators or not. This is that if
electrons with a determined energy and momentum can be trapped in the surface.
Real Space
Reciprocal space
Topological insulators
At bulk
At the surface new states appears!
Topological insulators
•Topological these two surfaces are equivalent
•However, the bulk properties of the semiconductor (or isolator) makes the surfaces
band to have spin-orbit coupling, so they stop being degenerated.
Topological insulators
•Depending of the properties of the bulk semiconductor (or the insulator), then the
surface bands are going to have the topological constrains.
•Now, what does make a topological insulator one? The fact that one electron with
certain energy and momentum would stay in that surface as it would with a conductor.
and this is going to be determined by the topology of the surface band!
•Let’s assume the red dot in the figure above is an electron from diffraction experiment,
on the left figure, the electron would bounce with different momentum from the solid.
However on figure on the right, the electron would get trapped.
Sources
• Nature Physics 4, 348 - 349 (2008)
doi:10.1038/nphys955
• Nature 464, 194-198 (11 March 2010) |
doi:10.1038/nature08916;