2 - Physics at Oregon State University
advertisement
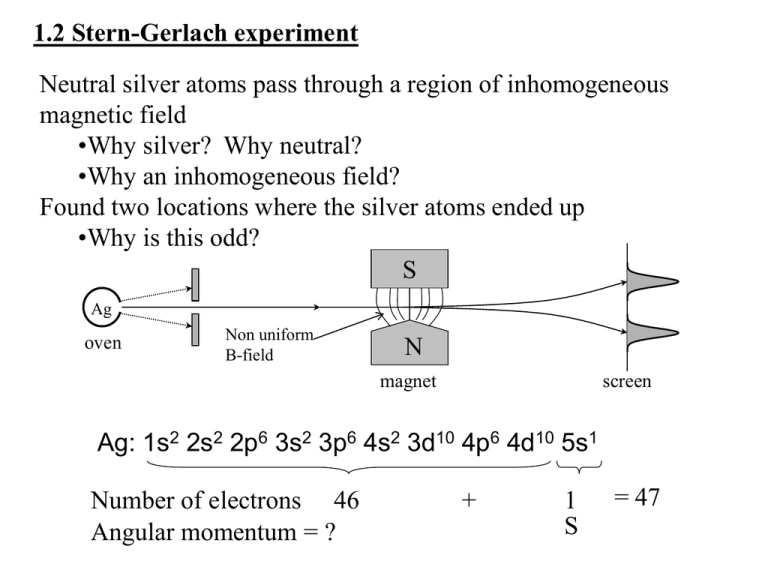
1.2 Stern-Gerlach experiment Neutral silver atoms pass through a region of inhomogeneous magnetic field •Why silver? Why neutral? •Why an inhomogeneous field? Found two locations where the silver atoms ended up •Why is this odd? S Ag oven Non uniform B-field N magnet screen Ag: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 4d10 5s1 Number of electrons 46 Angular momentum = ? + 1 S = 47 S-G historic overview • They thought there should be splitting with the Bohr model because they thought that the silver atom should have a h/2pi orbital angular momentum from that model, when in fact it's zero - L not zero, should see splitting (by S-G), and after much effort, they do and conclude Bohr is right - but why splitting and not uniform if initial orientations are random? (Einstein and Ehrenfest) - QM evolves, Bohr model found inadequate - wait, L IS zero, why did they see splitting? - then 5 years later, the idea of intrinsic spin... Consider our expectations on what should happen to a neutral particle in an inhomogeneous magnetic field: •What does a magnetic field interact with? •How can a neutral atom interact with a magnetic field? •Let’s derive it classically from intro-course principles •What does a simple magnetic dipole look like? •What does the energy look like? •What will the force be and why does the B need to be inhomogeneous? •How do we relate this to angular momentum? •Why do we introduce “spin”? •Does it really “spin”? •What is different between what we expect to observe classically and what we actually observe? •What is a projection? •What does two “spots” tell us about the spin? •What is quantization? “that” calculation • More rigorous details posted on blackboard • Found μ = (q/2m)L • Have such a term for orbital angular momentum L, “intrinsic spin” S, and for the total angular momentum (the QM sum of L and S) • Generalize: μ = g(q/2m)S – S is the “intrinsic angular momentum” – as if the electron spun on its axis, but NOT physical – g is the gyromagntic/gyroscopic/g-ratio – g is dimensionless – g for electron is one of best known values in physics What is “intrinsic spin”? • Also called “spin”, or spin angular momentum, or S • It’s a “degree of freedom”, or quantum number: a “state” the particle has • Does interact with magnetic fields like L, but not continuous! • NOT a physical rotation • INTRINSIC property – like charge and rest mass! We have no model for what “makes it up/causes it” for fundamental particles • Shows up most simply in Pauli exclusion principle