Odd Hydrogen, HOx - Atmospheric and Oceanic Science
advertisement

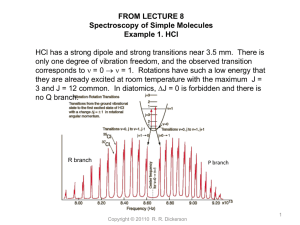
LECTURE 20 Atmospheric Odd Hydrogen, HOx AOSC 637 Spring 2010 Atmospheric Chemistry Russell R. Dickerson Copyright © 2010 R. R. Dickerson 1 Odd Hydrogen: Outline Importance Chemistry Sources Sinks Reservoirs and Ratios Detection Techniques Fluorescence FAGE DOAS Chem Amplification Global Budget Calculations Remaining Challenges Bibliography Odd Hydrogen • • • • Importance: Ozone destruction in both the stratosphere and trospsphere. Removal of NOx, ClOx, CO, VOC’s, SOx, HCFC’s The most important species for transformations. Many pollutants have no other sink. O3 h O2 O(1D) Chemistry Sources (remember what HCO and H do) ( 318 nm) H 2O O(1D) 2OH H 2 O(1D) OH H CH 4 O(1D) CH 3 OH CH 3OOH h CH 3O OH CH 3O O2 CH 2O HO2 CH O h CHO H Copyright © 20102 R. R. Dickerson 3 HYDROCARBRONS REACTIVITY FOR URBAN SMOG (OZONE) FORMATION HYDROCARBON k(O) k(O₃) k(OH) (All units: cm³s⁻¹) Methane, CH₄ 1.1x10⁻¹⁷ SLOW 7.9x10⁻¹⁵ Ethane, C₂H₆ 9.6x10⁻¹⁶ SLOW 2.7x10⁻¹³ Propane, C₃H₈ 1.5x10⁻¹⁴ SLOW 1.2x10⁻¹² Butane, C₄H₁₀ 3.1x10⁻¹⁴ SLOW 2.3x10⁻¹² Hexane, C₆H₁₄ 9.5x10⁻¹⁴ SLOW 5.7x10⁻¹² 2,3 Dimethyl butane (C₆H₁₄) 2.1x10⁻¹³ SLOW 6.3x10⁻ ¹² Ethene, C₂H₄ 8.4x10⁻¹³ 1.8x10⁻¹⁸ 8.0x10⁻¹² Propene, C₃H₆ 3.6x10⁻¹² 1.1x10⁻¹⁷ 2.5x10⁻¹¹ Benzene, C₆H₆ 1.6x10⁻¹⁴ SLOW 1.2x10⁻¹² 5.9x10⁻¹⁴ SLOW 6.4x10⁻¹² Toluene, C₇H₈ Copyright © 2010 R. R. Dickerson 4 Odd Hydrogen To calculate OH we need: j(O3), [O3], [H2O] Sinks OH OH H 2O O HO2 OH H 2O O2 M NO2 OH HNO3 (het _ rem oval) HO2 HO2 H 2O2 O2 OH HCl H 2O Cl ( strat) Copyright © 2010 R. R. Dickerson 5 Reservoir species, control ratios of OH/HO2 O2 OH CO CO2 HO2 HO2 NO NO2 HO HO2 O3 2O2 OH 2 / H 2O SO2 OH O H 2 SO4 HO2 OH H 2O2 HO2 H 2O OH H 2CO HCO H 2O OH VOC ' s HO2 RO2 HO2 RO2 HOOR O2 Copyright © 2010 R. R. Dickerson 6 RONO Copyright © 2010 2 R. R. Dickerson 7 Steady State Approximations: 2k2 [O(1D)][H 2O] [ HO2 ](k6 [O3 ] k7 [ NO]) 2 j9 [ H 2O2 ] [OH ] k3[CO] k 4 [CH 4 ] 1/ 2 ( j9 k10 [OH ] k11 )k2 [O( D)][H 2O] [ HO2 ] k8 (k10 [OH ] k11 ) 1 O3 h O2 O(1D)....(1) H 2O O( D) 2OH .............(2) 1 HO2 CO CO2 O2 .......(3) CH 4 OH CH 3 H 2O....(4) CH 3O O2 CH 2O HO2 ..(5) 2 HO2 H 2O2 O2 ............(8) H 2O2 h 2OH ..............(9) HO2 OH H 2O O2 ........(10) H 2O2 Heterog P roducts.......(11) HO2 O3 OH 2O2 .......(6) HO2 NO OH NO2 ......(7) From Logan, JGR (1981) Copyright © 2010 R. R. Dickerson 8 Calculated mean OH (x10-6 cm-3) in a CH4, CO, O3 atmosphere, from Crutzen’s model at MPI. Why does het max occur in the LFT in the tropics? Copyright © 2010 R. R. Dickerson 9 Potential Energy Curves for OH Chem. Phys. 237(1-2), 123-138 (1998) • DOI:10.1016/S0301-0104(98)00219-5 Copyright © 2010 R. R. Dickerson 10 Solar flux induced fluorescence was successful for the detection of OH in the Stratosphere (Anderson JGR, 7820, 1971). Anderson put a scanning spectrometer on the nose of a rocket and measured the emission at 308 nm due to solar excitation. In situ resonance fluorescence with a microwave discharge lamp worked in the strat (Anderson GRL, 1976), but not in the trop. Copyright © 2010 R. R. Dickerson 11 FAGE, Fluorescence Assay by Gas Expansion, is essentially laser-induced fluorescence at low pressure. Copyright © 2010 R. R. Dickerson 12 Early attempts to measure OH via fluorescence failed – why? Radiation at 308 nm photolyzes O3 to O(1D). Copyright © 2010 R. R. Dickerson 13 Copyright © 2010 R. R. Dickerson 14 Copyright © 2010 R. R. Dickerson 15 Diurnal variation of OH measured using LIF (o) and DOAS (•) during POPCORN (Adapted from Hofzumahaus et al., 1998). Copyright © 2010 R. R. Dickerson 16 Correlation plot of all LIF OH data versus the photolysis frequency of ozone, j(O1D). (Adapted from Holland et al., 1998). Copyright © 2010 R. R. Dickerson 17 Copyright © 2010 R. R. Dickerson 18 Atmospheric absorption spectra measured using DOAS as a function of time of day (UT). Solid lines are reference absorption spectra of OH radicals fitted to the measurements (Adapted from Dorn et al., 1996). Copyright © 2010 R. R. Dickerson 19 Copyright © 2010 R. R. Dickerson 20 Dependence of the measured OH concentration on NO2 during the POPCORN field campaign. To make this behavior visible, the OH data were first normalized with respect to j(O1D) and then plotted versus equal log(NO2)-intervals of 0.1. Full curve corresponds to the model-calculated dependence. [Adapted from Ehhalt, 1999]. Copyright © 2010 R. R. Dickerson 21 Comparison of observed and calculated OH concentrations versus NOX during the 1993 Idaho Hill experiment (THOPE). The different model calculations account for different amounts of unmeasured biogenic hydrocarbons [Adapted from McKeen et al., 1997]. Copyright © 2010 R. R. Dickerson 22 Altitude profiles of measured (open circles) and modeled OH for 10 May 1996 during SUCCESS. Measurements and models are averaged into 0.5 km altitude bins. Models with (dashdot line) and without (dashed line) acetone are compared. (Adapted from Brune et al., 1998). CH3C(O)CH3 + hv → 2CH3 + CO Copyright © 2010 R. R. Dickerson 23 Calculated mean OH (x10-6 cm-3) in a CH4, CO, O3 atmosphere, from Crutzen’s model at MPI. Why does het max occur in the LFT in the tropics? Copyright © 2010 R. R. Dickerson 24 Horowitz et al., JGR 2003. Copyright © 2010 R. R. Dickerson 25 Global mean OH can be calculated from the rate of loss of methyl chloroform, CCl3CH3 Prinn et Copyright © 2010 R. R. Dickerson al., Science, 1995. 26 Concentrations of CCl3CH3 continue to fall. Copyright © 2010 R. R. Dickerson 27 From NOAA Earth System Research Laboratory http://www.esrl.noaa.gov/gmd/ odgi/ Copyright © 2010 R. R. Dickerson 28 Calculating Global Mean OH from CH3CCl3 Concentrations Because OH is so hard to measure, we would like to get at the concentration another way [Prinn et al., 1987; Prinn et al., 1995]. Let’s designing a good experiment – a good OH tracer must have: 1. Only one sink – reaction with OH 2. A lifetime » inter-hemispheric mixing » seasonal variations in OH 1. Well known atmospheric burden 2. Well known production rate 3. Well known rate const, kOH 4. A reliable, precise measurement technique. Copyright © 2010 R. R. Dickerson 29 In the original work, Prinn performed a simple global burden calculation of mean [OH]. 1. Assume steady state, i.e., production = loss. 2. Measured or calculated production rate. 3. Loss (= production) = kOH [CH3CCl3][OH] 4. Assume [CH3CCl3] is constant in time and space (we’ll revisit this later). Production Prod [OH] Prod k OH [CH3CCl 3 ] k' Copyright © 2010 R. R. Dickerson 30 In the original work, Prinn performed a simple global burden calculation of mean [OH]. 1. Assume stready state, i.e., production = loss. 2. Measured or calculated production rate. 3. Loss (= production) = kOH [CH3CCl3][OH] 4. Assume [CH3CCl3] is constant in time and space. First order estimate (box model) CH3CCl3 + OH → H2O + CH2CCl3 kOH = 1.64x10-12e(-1520/T) Mean middle trop temp ~ 255 K; k255 = 4.2x10-15 cm3 s-1 Copyright © 2010 R. R. Dickerson 31 What was the mean [CH3CCl3]? Latutude Mixing Ratio (in 1981) 52°N 169 (ppt) 45°N 163 13°N 147 14°S 122 41°S 117 Lat weighted mean 144 ±25 ppt Total tropospheric burden = mass of atmosphere x mean mixing ratio x ratio of molecular weights. 4.0x1021 g x 1.44x10-10 x 133.5/29 = 2.65x1012 g From Prinn et al., (1983). Copyright © 2010 R. R. Dickerson 32 What was the mean [CH3CCl3]? The global production rate in 1981 was ~5.0x1011 (±0.5) g yr-1 We don’t know for sure that release = production. Lifeteime Burden 2.65x1012 5.3yr 11 Production 5x10 [OH] k 4.2x1015 x5.3x365x25x3600 1.4x106 cm3 1 Copyright © 2010 R. R. Dickerson 33 What was the uncertainty mean [OH] of 1.4x106 cm-3? 1. Rate Constant ±15% 2. Absolute concentration ± 20% 3. Production rate ± 10% 4. Mean global conc ± 25% 5. Annual variation ± 30% RMS ±50% Using a 12-box model, the global mean OH was estimated to be 9.7 ± 0.5x105 cm-3 in 1994 with little temporal change by Prinn et al., 1995. They also derived a residence timje for methylchloroform of 4.8 yr. Copyright © 2010 R. R. Dickerson 34 Remaining challenges I. II. III. What are the controlling factors in the upper trop.? Is the mean OH derived from methyl chloroform correct in light of recent discoveries about Cl chemistry in the trop? How will changes in the composition and climate impact the atmosphere’s oxidizing capacity and what unintended consequences await us? Copyright © 2010 R. R. Dickerson 35 Bibliography Anderson, J.G., The absolute concentration of OH (X2P) in the earth's stratosphere, Geophys. Res. Lett. 3, 165-168, 1976. Brune, W.H. et al., Airborne in-situ OH and HO2 observations in the cloud-free troposphere and lower stratosphere during SUCCESS, Geophys. Res. Lett., 25, 1701-1704, 1998. Ehhalt, D.H., Photooxidation of trace gases in the troposphere, Phys. Chem. Chem. Phys., 1, 5401-5408, 1999. McKeen et al., Photochemical modeling of hydroxyl and its relationship to other species during the Tropospheric OH Photochemistry Experiment, J. Geophys. Res., 102, 6467-6493, 1997. Dorn, H. P., U. Brandenburger, T. Brauers, M. Hausmann, and D. H. Ehhalt, In-situ detection of tropospheric OH radicals by folded long- path laser absorption. Results from the POPCORN field campaign in August 1994., Geophys. Res. Lett., 23, 2537-2540, 1996. Hard, T.M., L. A. George, and R. J. O'Brien, FAGE Determination of Tropospheric HO and HO2, J. Atmos. Sciences, 52, 3354-3372, 1995. Hausmann, M., U. Brandenburger, T. Brauers, and H.-P. Dorn, Detection of tropospheric OH radicals by long-path differential-optical-absorption spectroscopy: Experimental setup, accuracy, and precision, J. Geophys. Res., 102, 16011-16022, 1997. Logan, J. A., M. J. Prather, S. C. Wofsy, and M. B. McElroy (1981), Tropospheric chemistry: A global perspecvtive, J. Geophys. Res., 86, 7210-7254. Prinn, R., D. Cunnold, R. Rasmussen, P. Simmonds, F. Alyea, A. Crawford, P. Fraser, and R. Rosen (1987), Atmospheric trends in methylchloroform and the global average for the hydroxyl radical, Science, 238, 945-950. Prinn, R. G., R. F. Weiss, B. R. Miller, J. Huang, F. N. Alyea, D. M. Cunnold, P. J. Fraser, D. E. Hartley, and P. G. Simmonds (1995), Atmospheric Trends and Lifetime of CH3CCl3 and Global OH Concentrations, Science, 269, 187-192. Copyright © 2010 R. R. Dickerson 36