4-12-11 modern physics internet soln
advertisement

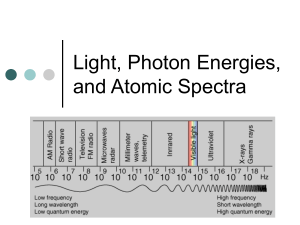
Modern Physics • What is meant by “quantized”? – Quantity • Specific and discrete quantity • Packets of definite size. • Quantized energy can be thought of as existing in very small packets of specific size. • Atoms absorb and emit quanta of energy. • Let us consider light… Electromagnetic Spectrum Visible spectrum Three models are used to describe light • What model is used in geometric optics, like with lenses and mirrors? – Ray • What model is used in studying diffraction and interference? – Wave • What model is used to study interaction of light and atoms? – Particle ( photon) But light is said to have a “dual nature” • What is that supposed to mean? – Wave particle duality • Waves have both a wave and a particle component • We describe the path of light as a ray – Equation • E = hf for a single photon • E = nhf for multiple photons –h = plank’s constant »6.63x10-34 J ∙ s (SI version) »4.14x10-15 eV ∙ s (convenient) –f = frequency –n = number of photons Conceptual checkpoint • Which has more energy in its photons, a very bright, powerful red laser or a small key-ring red laser? – Neither! They both have the same energy per photon. The big one has more power. • Which has more energy in its photons, a red laser or a green laser? – The green one has shorter wavelength and higher frequency. It has more energy per photon. The “electron-volt” (eV) • The electron-volt is the most useful unit on the atomic level. • If a moving electron is stopped by 1 V of electric potential, we say it has 1 electron-volt (or 1 eV) of kinetic energy. • 1 eV = 1.602×10-19 J • What is the frequency and wavelength of a photon whose energy is 4.0 x 10-19J? E = 4.0x10-19 J h= 6.625x10-34 J ∙ s E = hf f = E / h= 4.0x10-19 J / 6.625x10-34 J ∙ s = 6.04x1014 Hz λ = c/f λ = c/f = 3x108 m/s / 6.04x1014 1 / s = 4.97x10-7 m = 497 nm • How many photons are emitted per second by a HeNe laser that emits 3.0 mW of power at a wavelength of 632.8 nm? P = 3.0 mW = 0.003 W λ = 632.8 nm = 632.8x10-9 m P = E /t E = P ∙ t E = 0.003 W ∙ 1 s = 0.003 J f = c / λ = 3x108 m/s / 632.8x10-9 m = 4.74x1014 Hz E =n(hf) n =E / (hf) =0.0003 J / (6.625x10-34 J ∙ s ∙ 4.74x1014 Hz) = 9.55x1014 • What are atoms composed of? – Atoms consist of nuclei (protons and neutrons and electrons. • What happens when an atom encounters a photon? – The atom usually ignores the photon, but sometimes the atom absorbs the photon. • If the photon is absorbed by the atom, what happens next? – The photon disappears and winds up giving all its energy to the atom’s electrons. • This is a graph of energy levels for a hypothetical atom Atom loses an electron 0.0eV -1.0eV Highest energy level -3.0eV Higher energy levels. (Atoms has -5.5 eV to absorb energy to get from the ground state. Normal “unexcited” -11.5 eV state Ionization level Third excited state Second excited state First excited state Ground state (lowest energy level • What do we mean when we say the atoms energy levels are “quantized”? Ionization level 0.0eV •Only certain Third excited state -1.0eV energies are allowed. Second excited state -3.0eV •These are represented by the horizontal lines. -5.5 eV First excited state •The atom cannot exist at energies not shown in this graph! Ground state (lowest energy level -11.5 eV Absorption of photon by Atom • When a photon of light is absorbed by an atom, it causes an increase in the energy of the atom. • The photon disappears, and the energy of the atom increases by exactly the amount of energy contained in the photon. • The photon can be absorbed ONLY if it can produce an “allowed” energy increase in the atom. • Absorption of photon by atom 0.0eV Exited state E = hf -10.0eV ΔE = hf Ground state Absorption Spectrum • When an atom absorbs photons, it removes the photons from the white light striking the atom, resulting in dark bands in the spectrum. • Therefore, a spectrum with dark bands in it is called an absorption spectrum. • Absorption Spectrum 0.0eV Ionized Absorption spectra always involve atoms going up in energy level. -10.0eV Ground state Emission of photon by atom • When a photon of light is emitted by an atom, it causes a decrease in the energy of the atom. • A photon of light is created, and the energy of the atom decreases by exactly the amount of energy contained in the photon that is emitted. • The photon can be emitted ONLY if it can produce an “allowed” energy decrease in an excited atom. • Emission of photon by atom 0.0eV Exited state E = hf -10.0eV ΔE = hf Ground state Emission Spectrum • When an atom emits photons, it glows! The photons cause bright lines of light in a spectrum. • Therefore, a spectrum with bright bands in it is called an emission spectrum. • Emission of photon by atom 0.0eV Ionized Emission spectra always involve atoms going down in energy level. -10.0eV Ground state Photoelectric effect What is the frequency and wavelength of the light that will cause the atom shown to transition from the ground state to the first excited state? Draw the transition. h = 4.14x10-15 eV ∙ s ΔE = hf f = Δ E / h 0.0eV -1.0eV f = (11.5 – 3.0) / 4.14x10-15 -3.0eV 15 f = 1.45x10 Hz λ= c / f -5.5 eV λ= 3x108 / 1.45x1015 λ= 2.07x10-7 m λ= 207 nm Ionization level Third excited state Second excited state First excited state Ground state (lowest energy level -11.5 eV What is the longest wavelength of light that when absorbed will cause the atom shown to ionize from the ground state? Draw the transition. h = 4.14x10-15 eV ∙ s ΔE = hf f = Δ E / h 0.0eV -1.0eV f = (11.5 ) / 4.14x10-15 f= 2.78x1015 Hz λ= c / f -3.0eV -5.5 eV λ= 3x108 / 2.78x1015 λ= 1.08x10-7 m λ= 1-8 nm Ionization level Third excited state Second excited state First excited state Ground state (lowest energy level -11.5 eV The atom shown is in the second excited state . What frequencies of light are seen in its emission spectrum? Draw the transition. h = 4.14x10-15 eV ∙ s 1 ΔE = hf 0.0eV -1.0eV f=ΔE/h -3.0eV f = (11.5 – 3.0 ) / 4.14x10-15 f = 2.053x1015 Hz -5.5 eV Ionization level Third excited state Second excited state 1 2 First excited state 3 Ground state (lowest energy level -11.5 eV The atom shown is in the second excited state . What frequencies of light are seen in its emission spectrum? Draw the transition. h = 4.14x10-15 eV ∙ s 0.0eV -1.0eV 2 ΔE = hf f=ΔE/h f = (5.5-3 ) / 4.14x10-15 f = 6.09x1014 Hz -3.0eV Ionization level Third excited state Second excited state 1 -5.5 eV 2 First excited state 3 Ground state (lowest energy level -11.5 eV The atom shown is in the second excited state . What frequencies of light are seen in its emission spectrum? Draw the transition. h = 4.14x10-15 eV ∙ s 3 ΔE = hf 0.0eV -1.0eV f=ΔE/h f = (11.5-5.5 ) / -3.0eV -15 4.14x10 f = 1.45x1015 Hz -5.5 eV Ionization level Third excited state Second excited state 1 2 First excited state 3 Ground state (lowest energy level -11.5 eV The atom shown is in the second excited state . What frequencies of light are seen in its emission spectrum? Draw the transition. 1 Ionization level 15 f = 2.053x10 Hz 0.0eV Third excited state -1.0eV 2 Second excited state 14 f = 6.09x10 Hz -3.0eV 1 2 3 f =1.45x1015 Hz -5.5 eV First excited state 3 Ground state (lowest energy level -11.5 eV Atoms absorbing photons increase in energy •We’ve seen that if you shine light on atoms, they can Ionization level absorb photons and 0.0eV increase in energy. •The transition shown is the absorption of Photon -4.0eV an 8.0 eV photon by 4.0 eV with this atom. largest •You can use Planck’s allowed equation to calculate energy the frequency and wavelength of this Ground state photon. (lowest energy level -12 eV Question • Now, suppose a photon with TOO MUCH ENERGY encounters an atom? • If the atom is “photo-active”, a very interesting and useful phenomenon can occur… • This phenomenon is called the Photoelectric Effect. Photoelectric Effect Photon energy •Some “photoactive” metals can absorb photons that not only ionize the metal, but give Eph the electron enough kinetic 0.0eV energy to escape from the atom and travel away from it. •The electrons that escape are often called “photoelectrons”. •The binding energy or “work -4.0eV function” is the energy necessary to promote the electron to the ionization level. •The kinetic energy of the electron is the extra energy provided by the photon. -12 eV e- E = W0 + KE Kinetic energy Ionization level W0 = Work function Ground state (lowest energy level Photon energy Photoelectric Effect • Photon Energy = Work e- E = W0 + KE Function + Kinetic Energy E ph Kinetic energy • hf = Φ + Kmax • Kmax = hf – Φ Ionization level 0.0eV • K:max Kinetic energy of “photoelectrons” • hf: energy of the -4.0eV photon W0 = Work • Φ: binding energy or function “work function” of the metal. -12 eV Ground state (lowest energy level • Suppose the maximum wavelength a photon can have and still eject an electron from a metal is 340 nm. What is the work function of the metal surface? Kmax = hf – Φ Φ = hf 0 J = hf – Φ f=v/λ Φ = hv / λ Φ = (4.14x10-15eV ∙ 3x108 m/s) / 340x10-9 m Φ = 3.65 eV The longest wavelength is the lowest energy, and will provide no “extra” kinetic energy for the electron. Question • Suppose you collect Kmax and frequency data for a metal at several different frequencies. You then graph Kmax for photoelectrons on y-axis and frequency on x-axis. What information can you get from the slope and intercept of your data? Slope: Planck’s Constant Intercept: Φ - binding energy or “work function” The Photoelectric Effect experiment • The Photoelectric Effect experiment is one of the most famous experiments in modern physics. • The experiment is based on measuring the frequencies of light shining on a metal (which is controlled by the scientist), and measuring the resulting energy of the photoelectrons produced by seeing how much voltage is needed to stop them. • Albert Einstein won the Nobel Prize by explaining the results. Photoelectric Effect experiment Light Collector Metal (+) e- e- e- e- e- e- eee- e- V e- e- e- A e- e- eeeeee- e- Strange results in the Photoelectric Effect experiment • Voltage necessary to stop electrons is independent of intensity (brightness) of light. It depends only on the light’s frequency (or color). • Photoelectrons are not released below a certain frequency, regardless of intensity of light. • The release of photoelectrons is instantaneous, even in very feeble light, provided the frequency is above the cutoff. Voltage versus current for different intensities of light. Number of electrons (current) increases with brightness, but energy of electrons doesn’t! “Stopping Voltage” Vs, the voltage needed to stop the electrons, doesn’t change with light intensity. That means the kinetic energy of the electrons is ndependent of how bright the light is. Voltage versus current for different frequencies of light. Energy of electrons increases as the energy of the light increases. “Stopping Voltage” Vs changes with light frequency. That means the kinetic energy of the photoelectrons is dependent on light color. Experimental determination of the Kinetic Energy of a photoelectron • The kinetic energy of photoelectrons can be determined from the voltage (stopping potential) necessary to stop the electron. • If it takes 6.5 Volts to stop the electron, it has 6.5 eV of kinetic energy. Graph of Photoelectric Equation Kmax KMAX = hf - Φ y= mx + b Slope = h f Cut-off Frequency Φ (binding energy) • Question: Does a photon have mass? – A photon has a fixed amount of energy (E = hf). – We can calculate how much mass would have to be destroyed to create a photon (E=mc2). • Calculate the mass that must be destroyed to create a photon of 340 nm light. λ = 340x10-9 m h = 6.63x10-34 J∙s h = 6.63x10-34 (kg ∙m2 / s2)∙s E = hf E = mc2 hf = mc2 f=c/λ hc / λ = mc2 h / λ = mc m = h / (λc) m = 6.63x10-34 / (340x10-9 ∙3x108 ) = Momentum of a Photon • Does a photon have momentum? Yes • A photon’s momentum is calculated by – p = E / c = hf / c = h / λ We have experimental proof of the momentum of photons • Compton scattering – Proof that photons have momentum. – High-energy photons collided with electrons exhibit conservation of momentum. – Work Compton problems just like other conservation of momentum problems – except the momentum of a photon uses a different equation. • What is the frequency of a photon that has the same momentum as an electron with speed 1200 m/s? melectron = 9.11x10-31 Kg h = 6.63x10-34 J∙s p = mv h = 6.63x10-34 (kg ∙m2 / s2)∙s p = 9.11x10-31 Kg ∙ 12 m / s = p = hf / c f=Pc/h f = ( kg ∙ m/s) ∙ (3x108 m/s) / (6.63x10-34 (kg ∙m2 / s2)∙s) f = Wave-Particle Duality • Waves act like particles sometimes and particles act like waves sometimes. • This is most easily observed for very energetic photons (gamma or x-Ray) or very tiny particles (elections or nucleons) Particles and Photons both have Energy • A moving particle has kinetic energy – E = K = ½ mv2 • A particle has most of its energy locked up in its mass. – E = mc2 • A photon’s energy is calculated using its frequency – E = hf Particles and Photons both have Momentum • For a particle that is moving – p = mv – kg ∙ m/s • For a photon – p = h/λ – (kg ∙m2 / s2)∙s / m = kg ∙ m / s – Check out the units! They are those of momentum. Particles and Photons both have a Wavelength • For a photon – l = c/f • For a particle, which has an actual mass, this equation still works – λ= h/p where p = mv – This is referred to as the deBroglie wavelength We have experimental proof that particles have a wavelength • Davisson-Germer Experiment – Verified that electrons have wave properties by proving that they diffract. – Electrons were “shone” on a nickel surface and acted like light by diffraction and interference. – We’ll study diffraction in the next unit, and return to this experiment then… • What is the momentum of photons that have a wavelength of 620 nm? λ = 620x10-9 m h = 6.63x10-34 J∙s p = h / λ = 6.63x10-34 J∙s / 620x10-9 m = • What is the wavelength of a 2,200 kg elephant running at 1.2 m/s? m = 2200 kg v = 1.2 m / s p = mv = 2200 kg ∙1.2 m/s = p=h/λ λ = h / p = 6.63x10-34 J∙s / 12 m / s = Naming a Nucleus Mass number 12 6 Atomic number C • What are isotopes? • What characteristics do isotopes of the same element share? What characteristics do isotopes of the same element share? Atomic number What characteristics do isotopes of the same element not share? Atomic mass Radioactivity • • • • Naming Isotopes a Nucleus Isotopes have the same atomic number and different atomic mass. Isotopes have similar or identical chemistry. Isotopes have different nuclear behavior. Examples: 12 6 C C C 13 14 6 6 Elementary Particles mass 1 Proton charge 1 mass 1 Neutron charge 0 mass 0 Electron charge -1 p n e Negative chare 0 +1 e Positive chare Nuclear reactions • Nuclear Decay: a spontaneous process in which an unstable nucleus ejects a particle and changes to another nucleus. – Alpha decay – Beta decay • Beta Minus • Positron • Fission: a nucleus splits into two fragments of roughly equal size. • Fusion: Two nuclei combine to form another nucleus. Decay Reactions • Alpha decay – A nucleus ejects an alpha particle, which is just a helium nucleus. • Beta decay – A nucleus ejects a negative electron. • Positron decay – A nucleus ejects a positive electron. • Simulations – http://library.thinkquest.org/17940/texts/radioactivity /radioactivity.html Alpha (α) Decay Alpha particle (helium nucleus) is released. Alpha decay only occurs with very heavy elements. 239 94 Pu 235 92 U + 4 2 He Beta (β-)Decay A beta particle (negative electron) is released. Beta decay occurs when a nucleus has too many neutrons for the protons present. A neutron converts to a proton. An antineutrino is also released. 14 6 C 14 7 N + 0 -1 e +γ Beta (β+)Decay Positron (positive electron) is released. Positron decay occurs when a nucleus has too many protons for the neutrons present. A proton converts to a neutron. A neutrino is also released. 2 2 He 2 1 H + 0 1 e +γ Neutrino and Anti-Neutrino • Proposed to make beta and positron decay obey conservation of energy. • These particles possess energy and spin, but do not possess mass or charge. • They do not react easily with matter, and are extremely hard to detect. Gamma Radiation, γ •Gamma radiation is electromagnetic in nature. •Gamma photons are released by atoms which have just undergone a nuclear reaction when the excited new nucleus drops to its ground state. •The high energy in a gamma photon is calculated by E = hf. • Complete the reaction, identify the type of decay. 234 90 Th 234 Pa 91 + 0 e -1 + γ • Complete the reaction for the alpha decay of Thorium-232 232 90 Th 228 88 Ra + 4 2 He Nuclear Fission and Fusion Fission •Fission occurs when an unstable heavy nucleus splits apart into two lighter nuclei, forming two new elements. •Fission can be induced by free neutrons. •Mass is destroyed and energy produced according to E = mc2. • http://library.thinkquest.org/17940/texts/fission/fission.html • http://www.atomicarchive.com/Movies/index.shtml Neutron-induced fission • Neutron-induced fission produces a “chain reaction.” What does that mean? • Nuclear power plants operate by harnessing the energy released in fission in by controlling the chain reaction. • Nuclear weapons depend upon the initiation of an uncontrolled fission reaction. Critical Mass • The neutrons released from an atom that has undergone fission cannot immediately be absorbed by other nearby fissionable nuclei until they slow down to “thermal” levels. • How can this concept be used to explain why a chain reaction in nuclear fission will not occur unless a “critical mass” of the fissionable element is present at the same location? Nuclear Reactors •Nuclear reactors produce electrical energy through fission. •Advantages are that a large amount of energy is produced without burning fossil fuels or creating greenhouse gases. •A disadvantage is the production of highly radioactive waste. •Another simulation appears at http://www.howstuffworks.com/nuclear-power.htm Nuclear Weapons •Nuclear weapons have been used only twice, although they have been tested thousands of times. •Weapons based on nuclear fission involve slamming together enough material to produce an uncontrolled fission chain reaction. Little Boy was dropped on Hiroshima and contained U-235 produced in Oak Ridge, TN. Fission • Fission occurs only with very heavy elements, since fissionable nuclei are too large to be stable. • A charge/mass calculation is performed to balance the nuclear equation. • Mass is destroyed and energy produced according to E = mc2. • • • • Fusion Fusion occurs when two light nuclei come together to form a new nucleus of a new element. Fusion is the most energetic of all nuclear reactions. Energy is produced by fusion in the sun. Fusion of light elements can result in nonradioactive waste. Fusion •Fusion is the reaction that powers the sun, but it has not been reliably sustained on earth in a controlled reaction. •Advantages to developing controlled fusion would be the tremendous energy output and the lack of radioactive waste products. •Disadvantages are – we don’t know if we’ll be technically able to do it on earth! Mass defect • This strange term is used to indicate how much mass is destroyed when a nucleus is created from its component parts. • The mass defect is generally much, much less than the mass of a proton or neutron, but is significant nonetheless. • The loss of mass results in creation of energy, according to E = mc2. 89