Coulomb`s Law
advertisement

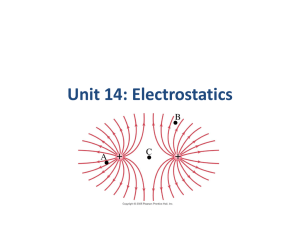
Unit 14: Electrostatics Units of Chapter 16 • Static Electricity; Electric Charge and Its Conservation • Electric Charge in the Atom • Insulators and Conductors • Induced Charge; the Electroscope • Coulomb’s Law • Solving Problems Involving Coulomb’s Law and Vectors • The Electric Field Units of Chapter 16 • Field Lines • Electric Fields and Conductors Do Now 1. How do protons and electrons differ in their electrical charge? 2. Is an electron in a hydrogen atom the same as an electron in a uranium atom? 3. Which has more mass – a proton or an electron? 4. In a normal atom, how many electrons are there compared to protons? Do Now 1. How do protons and electrons differ in their electrical charge? Same magnitude, but opposite charge. 2. Is an electron in a hydrogen atom the same as an electron in a uranium atom? Yes. 3. Which has more mass – a proton or an electron? Proton 4. In a normal atom, how many electrons are there compared to protons? Same number, no net charge. Atomic Structure 16.1 Static Electricity; Electric Charge and Its Conservation Objects can be charged by rubbing Triboelectric Series • Friction can cause electrons to transfer from one material to another • Different materials have a different degree of attraction for electrons • The triboelectric series determines which materials have a greater attraction. • When two materials are rubbed together, the one with the higher attraction will end up getting some of the electrons from the other material Triboelectric Series If two materials are rubbed together, the one that is higher in the series will give up electrons and become more positive. MORE POSITIVE Human Hands (if very dry) Leather Rabbit Fur Glass Human Hair Nylon Wool Fur Lead Silk Aluminum Paper Cotton Steel (neutral) Wood Amber Hard Rubber Nickel, Copper Brass, Silver Gold, Platinum Polyester Styrene (Styrofoam) Saran Wrap Polyurethane Polyethylene (scotch tape) Polypropylene Vinyl (PVC) Silicon Teflon MORE NEGATIVE Question • If fur is rubbed on glass, will the glass become positively charged or negatively charged? • Glass – positive • Fur -negative 16.1 Static Electricity; Electric Charge and Its Conservation Charge comes in two types, positive and negative; like charges repel and opposite charges attract Interaction Between Charged and Neutral Objects • Any charged object - whether positively charged or negatively charged - will have an attractive interaction with a neutral object. 16.1 Static Electricity; Electric Charge and Its Conservation Electric charge is conserved – the arithmetic sum of the total charge cannot change in any interaction. 16.2 Electric Charge in the Atom Atom: Nucleus (small, massive, positive charge) Electron cloud (large, very low density, negative charge) 16.2 Electric Charge in the Atom Atom is electrically neutral. Rubbing charges objects by moving electrons from one to the other. 16.2 Electric Charge in the Atom Polar molecule: neutral overall, but charge not evenly distributed 16.3 Insulators and Conductors Conductor: Insulator: Charge flows freely Almost no charge flows Metals Most other materials Some materials are semiconductors. How Charge Is Transferred • Objects can be charged by rubbing • Metal objects can be charged by conduction • Metal objects can be charged by • induction 16.4 Induced Charge; the Electroscope Metal objects can be charged by conduction: Charging by Induction • When an object gets charged by induction, a charge is created by the influence of a charged object but not by contact with a charged object. The word induction means to influence without contact. • If a rubber balloon is charged negatively (perhaps by rubbing it with animal fur) and brought near (without touching) the spheres, electrons within the two-sphere system will be induced to move away from the balloon. Charging By Induction With Negatively Charged Object In step iii, why is the charge on the right sphere almost uniformly distributed? Charging By Induction With Negatively Charged Object What was the source of positive charge that ended up on sphere B? Source of charge in induction • In induction, the source of charge that is on the final object is not the result of movement from the charged object to the neutral object. Ground • An infinite source or sink for charge 16.4 Induced Charge; the Electroscope They can also be charged by induction: 16.4 Induced Charge; the Electroscope The electroscope can be used for detecting charge: 16.4 Induced Charge; the Electroscope The electroscope can be charged either by conduction or by induction. 16.4 Induced Charge; the Electroscope The charged electroscope can then be used to determine the sign of an unknown charge. 16.4 Induced Charge; the Electroscope Nonconductors won’t become charged by conduction or induction, but will experience charge rearrangement. The atoms or molecules become polarized. : 16.4 Induced Charge; the Electroscope Nonconductors won’t become charged by conduction or induction, but will experience charge separation: Do Now True or False? Explain your reasoning. 1. An object that is positively charged contains all protons and no electrons. False Positively charged objects have electrons; they simply possess more protons than electrons. 2. An object that is electrically neutral contains only neutrons. False Electrically neutral atoms simply possess the same number of electrons as protons. Do Now True or False? Explain your reasoning. 1. An object that is positively charged contains all protons and no electrons. 2. An object that is electrically neutral contains only neutrons. Coulomb’s Law • The French physicist Charles Coulomb (1736 – 1806) investigated electric forces in the 1780s using a torsion balance. 16.5 Coulomb’s Law Experiment shows that the electric force between two charges is proportional to the product of the charges and inversely proportional to the distance between them. Coulomb’s Law 1. If two point charges Q 1 and Q 2 are a distance r apart, the charges exert forces on each object of magnitude: Q1 F1 on 2 F 2 on 1 k Q2 r 2 These forces are an action/reaction pair, equal in magnitude but opposite in direction. 16.5 Coulomb’s Law 2. The forces are along the line connecting the charges, and is attractive if the charges are opposite, and repulsive if they are the same. 16.5 Coulomb’s Law Unit of charge: coulomb, C The proportionality constant in Coulomb’s law is then: When we only need two significant figures: k 9 . 0 10 N m / C 9 2 2 Charges produced by rubbing are typically around a microcoulomb: 16.5 Coulomb’s Law Charge on the electron: Electric charge is quantized in units of the electron charge. This is the smallest charge in nature – fundamental or elementary charge. The net charge of any object must be a multiple of that charge. 16.5 Coulomb’s Law The proportionality constant k can also be written in terms of , the permittivity of free space: (16-2) 16.5 Coulomb’s Law Coulomb’s law strictly applies only to point charges. Superposition: for multiple point charges, the forces on each charge from every other charge can be calculated and then added as vectors. 16.6 Solving Problems Involving Coulomb’s Law and Vectors The net force on a charge is the vector sum of all the forces acting on it. 16.6 Solving Problems Involving Coulomb’s Law and Vectors Vector addition review: Example 1 Suppose that two point charges, each with a charge of +1.00 Coulomb are separated by a distance of 1.00 meter. Determine the magnitude of the electrical force of repulsion between them. Given: Find: F - ? IQI I= 1.00 C Q2 I= 1.00 C r = 1.00 m Solve: F k Q1 Q 2 r 2 ( 9 . 0 10 )(1)(1) 9 F 1 2 9 . 0 10 N 9 The force of repulsion of two +1.00 Coulomb charges held 1.00 meter apart is 9 billion Newton. This is an incredibly large force that compares in magnitude to the weight of more than 2000 jetliners. Example 2 Determine the magnitude and direction of the electric force on the electron of a hydrogen atom exerted by the single proton that is the atom’s nucleus. Assume the average distance between the revolving 10 electron an the proton r 0 . 53 10 m Given: Find: F-? Q 1 Q 2 1 . 6 10 r 0 . 53 10 10 19 m C Solution: F k Q1 Q 2 r 2 ( 9 . 0 10 N m / C )(1 . 6 10 9 2 2 ( 0 . 53 10 10 19 m) 2 C )(1 . 6 10 19 C) 8 . 2 10 8 N Problem 1 A balloon with a charge of 4 . 0 10 5 C is held a distance of 0.10m from a second balloon having the same charge. Calculate the magnitude of the electrical force between the charges. Draw a diagram. F k Q1 Q 2 r 2 ( 9 . 0 10 N m / C )( 4 . 0 10 9 2 2 ( 0 . 10 m ) 2 5 C )( 4 . 0 10 5 C) 14400 10 1 1440 N Problem 2 • Calculate the electrical force exerted between a 22-gram balloon with a charge of -2.6μC and a wool sweater with a charge of +3.8μC; the separation distance is 75cm. (Note: 1 C 1 10 C ) 6 F k Q1 Q 2 r 2 ( 9 . 0 10 N m / C )( 2 . 6 10 9 2 2 ( 0 . 75 m ) 2 6 C )( 2 . 6 10 6 C) 158 . 08 10 3 N 0 . 158 N Neutral vs. Charged Objects • if an atom contains equal numbers of protons and electrons, the atom is described as being electrically neutral. • if an atom has an unequal number of protons and electrons, then the atom is electrically charged and referred to as an ion. • Any particle, whether an atom, molecule or ion, that contains less electrons than protons is said to be positively charged. • Conversely, any particle that contains more electrons than protons is said to be negatively charged. Charged Objects as an Imbalance of Protons and Electrons • Electrons can move to the electrons’ shells of other atoms of different materials. • For electrons to make a move from the atoms of one material to the atoms of another material, there must be an energy source and a low-resistance pathway. • rubbing your feet on carpet • clothes tumble in the dryer Charged Objects as an Imbalance of Protons and Electrons • The principle stated earlier for atoms can be applied to objects. • Objects with more electrons than protons are charged negatively; objects with fewer electrons than protons are charged positively. True or False? 1. An object that is positively charged contains all protons and no electrons. False Positively charged objects have electrons; they simply possess more protons than electrons. 2. An object that is electrically neutral contains only neutrons. False Electrically neutral atoms simply possess the same number of electrons as protons. Do Now Suppose you rub a glass rod with a silk cloth and a second glass rod with rabbit fur. The silk cloth will acquire a __________ (+ , -) charge; the rabbit fur will acquire a __________ (+ , -) charge. The rabbit fur and the silk cloth will then be observed to ______________________ (attract, repel, not interact with) each other. Three Ways to Charge an Object 1. Friction (by rubbing) 2. Conduction(with contact) 3. Induction(without contact) Ground An infinite source or sink for charge • Charge always distributes itself evenly around a conducting sphere • We can think of ground as a conductor that is so large that it can always accept more charge (or provide more charge). Symbol Charging by Conduction • When charging something by contact it is important to note the following properties • The objects must actually touch and transfer some electrons. • The objects become charged alike. • The original charged object becomes less charged because it actually lost some charge. The Electroscope The electroscope can be used for detecting charge: Electroscope Can be Charged by Induction Electroscope Can be Charged by Conduction • Once the contact of the rod to the electroscope is made, the electrons move from the electroscope to the rod. The electroscope is positively charged. Nonconductors Nonconductors won’t become charged by conduction or induction, but will experience charge rearrangement. The atoms or molecules become polarized. Do Now • Object A is rubbed with object B. Object C is rubbed with • object D. Objects A and D are observed to repel each other. • Object B is observed to repel a negatively charged balloon. • This is conclusive evidence that … • … object A acquired a __________ (+ , -) charge. • … object B acquired a __________ (+ , -) charge. • … object C acquired a __________ (+ , -) charge. • … object D acquired a __________ (+ , -) charge. Law of Universal Gravitation Analogy • Coulomb’s Law F k q1 q 2 d 2 • Law of Universal Gravitation F G m1m 2 d 2 Questions: 1. Two charged objects have a repulsive force of .080 N. If the charge of one of the objects is doubled, then what is the new force? 2. Two charged objects have a repulsive force of .080 N. If the distance separating the objects is doubled, then what is the new force? Do Now • • • • Object A is rubbed with object B. Object C is rubbed with object D. Objects A and D are observed to repel each other. Object B is observed to repel a negatively charged balloon. This is conclusive evidence that … … object A acquired a __________ (+ , -) charge. … object B acquired a __________ (+ , -) charge. … object C acquired a __________ (+ , -) charge. … object D acquired a __________ (+ , -) charge. Six Flags Trip 1. Meet in the auditorium after Pd.2. 2. 1PM Check in at buffet in Old Country Picnic Grove. 3. 5 PM Meet at fountain. 4. Cell phone. 5. Sunscreen. Do Now • Find the force exerted on the test charge. Indicate the direction of that force (Hint: Calculate individual forces on the test charge and add them as vectors.) qA= +2nC 1m qtest=-1C 1m qB=+3nC Solution F AonT k Q AQT r 2 ( 9 10 )( 2 10 9 (1) 9 )(1) 2 18 N to the left F BonT k Q B QT r 2 ( 9 10 )( 3 10 9 F net 27 N 18 N 9 N (1) 9 2 to the right )(1) 27 N to the right Gravitational Force • Objects near surface of Earth Force • Gravitational force always directed towards the center of Earth. GMm F mg r r g GM r 2 2 Gravitational Force Force depends on mass of object. F mg Gravitational force always directed towards center of earth. Even when there is no mass nearby the earth, we can still talk about a gravitational field near the earth- pointing towards earth. Electric Field • Space around Earth and every mass is filled with gravitational field. • Space around every electric charge is filled with electric field. Strength of Electric Field • An electric field is a vector. It has both magnitude and direction. • Its magnitude can be measured by its effect on a small positive test charge q placed in it. • The greater the force acting on the charge, the stronger the electric field. • E – strength of electric field E F electric q Units – N/C 16.7 The Electric Field The electric field is the force on a small (point) charge, divided by the charge: • If you know the direction and magnitude of the electric field, you can determine the direction of the force. • Negatively charged particles will have opposite direction of force. The Electric Field E F /q F kqQ r F q q- test charge 2 Q – source charge kQ r 2 General Expression for point charge: E k Q r 2 Gravitational Field Analogy • Strength of electric field: E F electric q • Strength of gravitational field: F=mg g F gravitatio m nal Example 1 Jack pulls his wool sweater over his head, which charges his cotton t-shirt as the sweater rubs against it. a) What is the magnitude and direction of the 19 1 . 60 10 C electric field at a location where a -piece of lint experiences a force of 9 3 . 2 10 N as it floats near Jack? Given: q 1 . 60 10 19 C F 3 . 2 10 9 N • Solution: E F q 3 . 2 10 1 . 60 10 9 N 19 C 2 . 0 10 10 N /C Example 2 Charge Q acts as a point charge to create an electric field. Its strength, measured a distance of 30 cm away, is 40 N/C. What would be the electric field strength ... a)30 cm away from a source with charge 2Q? b)60 cm away from a source with charge Q? a) 80N/c Q E k 2 b) 10N/C r Example 3 What is the magnitude and direction of the electric field 0.25 meters away from a source charge with -5.0 μC. Draw a diagram. E kQ r 2 ( 9 10 )( 5 . 0 10 9 ( 0 . 25 ) 2 6 ) 7 . 2 10 N / C 5 16.7 The Electric Field Force on a point charge in an electric field: (16-5) Superposition principle for electric fields: 16.7 The Electric Field Problem solving in electrostatics: electric forces and electric fields 1. Draw a diagram; show all charges, with signs, and electric fields and forces with directions 2. Calculate forces using Coulomb’s law 3. Add forces vectorially to get result 16.8 Field Lines The electric field can be represented by field lines. These lines start on a positive charge and end on a negative charge. 16.8 Field Lines The number of field lines starting (ending) on a positive (negative) charge is proportional to the magnitude of the charge. The electric field is stronger where the field lines are closer together. 16.8 Field Lines Electric dipole: two equal charges, opposite in sign: 16.8 Field Lines The electric field between two closely spaced, oppositely charged parallel plates is constant. 16.8 Field Lines Summary of field lines: 1. Field lines indicate the direction of the field; the field is tangent to the line. 2. The magnitude of the field is proportional to the density of the lines. 3. Field lines start on positive charges and end on negative charges; the number is proportional to the magnitude of the charge. 16.9 Electric Fields and Conductors The static electric field inside a conductor is zero – if it were not, the charges would move. The net charge on a conductor is on its surface. Faraday’s Cage, Shielding A conducting box used for shielding delicate instruments from unwanted external electric fields. 16.9 Electric Fields and Conductors The electric field is perpendicular to the surface of a conductor – again, if it were not, charges would move. 16.12 Photocopy Machines and Computer Printers Use Electrostatics Laser printer is similar, except a computer controls the laser intensity to form the image on the drum Summary of Chapter 16 • Two kinds of electric charge – positive and negative • Charge is conserved • Charge on electron: • Conductors: electrons free to move • Insulators: nonconductors Summary of Chapter 16 • Charge is quantized in units of e • Objects can be charged by conduction or induction • Coulomb’s law: • Electric field is force per unit charge: Summary of Chapter 16 • Electric field of a point charge: E kQ r 2 • Electric field can be represented by electric field lines • Static electric field inside conductor is zero; surface field is perpendicular to surface Chapter 17 Electric Potential Units of Chapter 17 • Electric Potential Energy and Potential Difference •Relation between Electric Potential and Electric Field •Equipotential Lines •Capacitance 17.1 Electrostatic Potential Energy and Potential Difference The electrostatic force is conservative – potential energy can be defined Change in electric potential energy is negative of work done by electric force: (17-1) Electric Potential ElectricPo tential Electrical PotentialE nergy Ch arg e 1volt 1 joule coulomb 17.1 Electrostatic Potential Energy and Potential Difference Electric potential is defined as potential energy per unit charge: (17-2a) Unit of electric potential: the volt (V). 1 V = I J/C. 17.1 Electrostatic Potential Energy and Potential Difference Only changes in potential can be measured, allowing free assignment of V = 0. (17-2b) Example 4 What is the potential difference between the terminals of a battery if 60.J of work are done when 3.0 C are pushed trough a wire from one terminal to the other? Given: W=60J q= 3.0C Find: V-? Solution: V W q 60 . J 3 .0 C 20 .V 17.1 Electrostatic Potential Energy and Potential Difference Analogy between gravitational and electrical potential energy: Electrical Potential Energy • Work is done when you move an object in the direction of the force. • When you raise an object, you move it against gravitational field, you increase its potential energy. • When you move electric charge against electric field, you increase its potential energy. Diagram B. Diagram A. • Work would not be • Moving a positive test charge required to move an against the direction of an object from a high electric field is like moving a potential energy mass upward within Earth's location to a low gravitational field. potential energy • Both movements would be like location. going against nature and would require work by an external force. 17.2 Relation between Electric Potential and Electric Field Work is charge multiplied by potential: Work is also force multiplied by distance: 17.2 Relation between Electric Potential and Electric Field Solving for the field, (17-4b) If the field is not uniform, it can be calculated at multiple points: 17.3 Equipotential Lines An equipotential is a line or surface over which the potential is constant. Electric field lines are perpendicular to equipotentials. The surface of a conductor is an equipotential. Equipotential Lines • Equipotential lines are like contour lines on a map which trace lines of equal altitude. In this case the "altitude" is electric potential or voltage. • Equipotential lines are always perpendicular to the electric field. • Movement along an equipotential line requires no work because such movement is always perpendicular to the electric field. 17.3 Equipotential Lines Equipotential Lines • Dashed lines are equipotential lines • Solid lines are electric field lines Summary of Chapter 17 • Electric potential energy: • Electric potential difference: work done to move charge from one point to another • Relationship between potential difference and field: Summary of Chapter 17 • Equipotential: line or surface along which potential is the same •