PES ppt
advertisement

Photoelectron
Spectroscopy
Straightforward evidence for
the validity of orbital diagram
Konsler 2013
Electron Configuration
Is there any direct
evidence that this
diagram is
accurately showing
potential energy of
electrons on the
atom?
Some Available Evidence
• Atomic Emission Spectra
• Successive Ionization
• Photoelectron Spectroscopy
The topic is probably best introduced in1st year
Chemistry under Atomic Electronic Structure
Atomic Emission Spectra
Atomic emission spectra are evidence that there are
specific allowed PE values by showing the energy
difference between some of them.
Limitations
Differences between PE values, not the values themselves.
Ambiguous origin and destination orbitals.
Impossible to visually compare PE of orbitals on multiple
atoms; large data sets will require many calculations.
Successive Ionization
Using successive ionization it
is usually possible to
determine the number of
valence electrons an atom
has. This is evidence that our
PE diagram is an accurate
representation
Limitations
Each ionization causes a
reorganization of the remaining
electrons, meaning the successive
ionization is not a measurement of
the characteristics of the original
atom. We infer there is a
relationship between the ions and
the parent atom.
Limitations
It’s not feasible to do more
than 5 successive ionizations,
making core electrons (and
sometimes even valence
electrons) impossible to obtain
values for.
Limitations
It’s not possible to determine if
electrons at the same energy
level on the atom have the
same PE initially, because we
remove them one at a time. As
a result, the method is really a
support only for n, not the
other 3 quantum numbers.
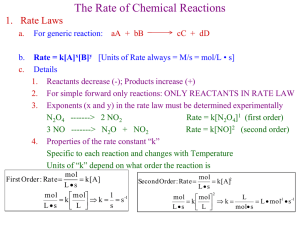
Photoelectron Spectroscopy
Ephoton = hv
Atom
Monochromatic
Beam of X-Rays
IEelectron = Ephoton - KE
Each event happens once for a
single atom. This is a quantum
event. Since Ephoton > IEelectron for
all the electrons on the atom, the
electron removed is random and
the KE of the electron is a
characteristic of that electron as
it exists on the atom at the
moment of the event.
KE = mv2
2
-
e
Negatively Charged Hemisphere (Constant V)
Retarding Voltage is directly
proportional to KEelectron
Independent
Variable (x)
Retarding
Voltage of Lens
Negatively
Charged
Hemisphere
(Constant V)
Dependent
Variable (y)
Intensity
Beam of Atoms
Electron Detector
Electrons scan past detector
Electrostatic Lens
(Focuses and slows electrons)
The intensity is measured as the retarding voltage on
the lens is reduced at a constant rate
X-Rays
(monochromatic)
Electrons ejected
Photoelectron Spectroscopy is still limited by Ephoton. If IEelectron is
too large, the electron will not be detected. This means that
electrons with low values of n on atoms with high value of Z may
be off the left hand side of the chart.
High Ionization Energy
Low electron PE
20 MJ/mol
Low Ionization Energy
High electron PE
10 MJ/mol
IEelectron = Ephoton - KEelectron
0 MJ/mol
However, a fluorescence source emits X-Rays with energy of over
1000 eV, several times greater than for successive ionization
instrumentation. In addition, core electrons are removed from a
neutral atom, requiring less energy.
High Ionization Energy
Low electron PE
20 MJ/mol
Low Ionization Energy
High electron PE
10 MJ/mol
IEelectron = Ephoton - KEelectron
0 MJ/mol
Photoelectron Spectrum
Helium possesses the valence electron with the
lowest PE of all elements. The measured value for
He is 2.37 MJ/mol. Any values observed with greater
values (to the left of this line) must be core electrons.
Note, d-subshell core electrons will be to the right of
this line.
20 MJ/mol
10 MJ/mol
Valence
0 MJ/mol
“Valence Line”: He(1s) = 2.37 MJ/mol
Photoelectron Spectrum
Relative Intensity = 2
Since each event removes a random electron from
a separate atom, the relative intensity shows the
proportion of electrons at that PE.
Relative Intensity = 1
20 MJ/mol
10 MJ/mol
0 MJ/mol
Photoelectron Spectrum
Valence
19.3 MJ/mol
RI = 2
1.36 MJ/mol
RI = 2
0.80 MJ/mol
RI = 1
20 MJ/mol
10 MJ/mol
0 MJ/mol
Photoelectron Spectrum
19.3 MJ/mol
RI = 2
1s2
20 MJ/mol
Boron (Z=5)
Analysis:
1) Valence has 2 values:
2) RI is 2 to 1 in valence:
3) Closest core has RI 2 not 6:
4) s2s2p1 must be 1s22s22p1
10 MJ/mol
sp
s2p1
not pxs2p1
1.36 MJ/mol
RI = 2
2s2
0.80 MJ/mol
RI = 1
2p1
0 MJ/mol
Photoelectron Spectrum
2p6
3.67 MJ/mol
1s2
2s2
104 MJ/mol
Sodium (Z=11)
3s1
6.84 MJ/mol
0.50 MJ/mol
{}
8 MJ/mol
4 MJ/mol
0 MJ/mol
But there aren’t even any instruments!
•
•
•
All PES spectra will probably be “simulated” for the following reasons:
PES post-dates atomic theory.
Most PES is a solid-phase technique. Having so many nearby atoms will
cause interference. For example, an electron promoted out of a low lying
orbital will leave a vacancy which can be occupied by an outer orbital electron
with concomitant production of a photon. If that photon is of high enough
energy, it might eject a more easily ionized electron in the same atom or a
nearby atom. This will result in the production of background interference and
even spurious peaks. As a result, PES spectra derived from solids would be
impossible for a non-expert to interpret.
Why do it?
•
•
•
•
Although it requires “simulated spectra” it is superior to other methods we describe
in General Chemistry because, even in principle, atomic emission and successive
ionization do not provide data which could be used to identify an atom in the context
of an exam. At best they would allow a student to choose the most likely among a
set of possibilities.
Photoelectron spectroscopy allows questions to be written using visual as opposed
to tabular data (as is required for successive ionization).
Students can be asked to interpret a spectrum but they can also legitimately be
asked to draw one. It’s a decently easy grade if you give the axes and requires
essentially no “give away” information in the stimulus.
Therefore, while the instruments aren’t accessible, understanding the principle
allows more thoughtful questions to be asked about electronic structure than were
previously possible.