AP Quantum Quiz Review
advertisement

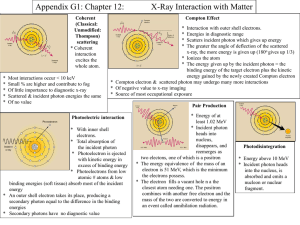
Do Now (3/6/14): • What are the major topics in our Quantum unit? AP Quantum Quiz Review 3/6/14 AP Test Review Competition • Winning group gets extra credit #1 What is the term used to refer to the minimum energy required for a photoelectron to escape from a metal plate in a photocell? a. Stopping voltage b. Planck’s constant c. Threshold wavelength d. Work function #2 • According to Einstein, the energy of a photon depends on the ____________ of the electromagnetic radiation. a. Momentum b. Speed c. Frequency d. Intensity #3 What will likely happen if a light whose frequency is below the threshold frequency hits a clean metal surface? a. No electron will be ejected from the metal b. Fewer electrons will be ejected from the metal c. More electrons will be ejected from the metal d. Ejected electrons will have a higher kinetic energy #4 • When light is directed on a metal surface, the kinetic energies of the electron: a. Vary with the intensity of light b. Vary with the frequency of light c. Vary with the speed of light d. Are random #5 • Threshold frequency is to work function as hertz is to which one of the following? a. Coulomb b. Joule c. Newton d. Watt #6 • The variable that varies directly with the amount of current produced by the photoelectrons is: a. The intensity of the incident light b. The frequency of the incident light c. The wavelength of the incident light d. The work function of the metal surface #7 • The threshold frequency has a value of X. If the frequency of the incident light increase from 2X to 4X, then the resulting current of photoelectrons: a. Is doubled b. Is increased by a factor of 3 c. Is reduced by half d. Remains the same #8 • A scientist is trying to eject electrons from a metal by shining a light on it, but none are coming out. To eject electrons, she should change the light by… a. Decreasing the frequency b. Increasing the frequency c. Increasing the intensity d. Increasing the wavelength #9 • Which of the following statements is true about a photon? a. A photon has zero mass and zero momentum b. A photon has finite mass and a finite value of momentum c. A photon has zero mass and a finite value of momentum d. A photon has finite mass but zero momentum #10 The work function of sodium is greater than that of potassium. If both the surfaces are irradiated with photons of the same wavelength, then the KE of the photoelectrons in the sodium surface as compared to the KE of the photoelectrons in the potassium surface will be: a. Same b. Less c. More d. Cannot be determined #11 In the Compton scattering experiment with X-rays incident upon a carbon block, what happens to the shift in wavelength as the scattering angle becomes larger? a. Increases b. Decreases c. Remains constant d. Increases until it reaches maximum at 90° #12 In the Compton scattering experiment, what happens to the energy lost by the incident photon? a. It becomes the kinetic energy of the target electron b. It is emitted as a photon with higher frequency c. It is emitted as a photon with lower frequency d. It is absorbed by the target electron, causing the electron to jump to an excited state #13 The collision between a photon and a free electron was first explained by which of the following scientists? a. Einstein b. Heisenberg c. Compton d. Bohr #14 • In the Compton Effect, a photon of wavelength λ collides with a stationary electron. The wavelength of the emitted photon is: a. Longer than λ b. Shorter than λ c. The same as λ d. No photon is emitted #15 • Which of the following objects would have the longest de Broglie wavelength if they were moving at the same speed? a. Ping pong ball b. Bowling ball c. Bicycle d. Bus #16 Which of the following statements correctly describes the de Broglie wavelength? a. The higher the momentum of the object, the longer the de Broglie wavelength b. The lower the momentum of the object, the longer de Broglie wavelength c. The more mass the object has, the longer the de Broglie wavelength d. The faster the object moves, the longer the de Broglie wavelength #17 • According to de Broglie, the waves are associated with: a. Moving charged particles only b. Moving neutral particles only c. All particles d. All moving particles #18 • The de Broglie wavelength of a particle is given by: a. h+mv b. hmv c. h/mv d. mv/c e. mv #20 A photon of energy E0 strikes a free electron, with the scattered photon energy E moving in the direction opposite that of the incident photon. In this Compton effect interaction, the resulting kinetic energy of the electron is: a. E0 b. E c. E0-E d. E0+ E e. Not given #21 A photon of energy E0 strikes a free electron, with the scattered photon energy E moving in the direction opposite that of the incident photon. In this Compton effect interaction, the resulting kinetic energy of the electron is: a. E0/c b. < E0/c c. > E0/c d. (E0- E)/c e. (E- E0)/c #22 Five photons have the following energy values. Which one represents the visible light photon? (a) 24.8 eV (b)12.4 eV (c) 6.2 eV (d) 2.48 eV (e) 1.24 eV #22 • D. Photons in the visible region have wave length range from 4000 Ǻ to 7000 Ǻ (approximately). The product of the energy in eV and the wave length in Angstrom in the case of any photon is 12400 (very nearly). • The energy of the 4000 Ǻ photon in electron volt is 12400/4000 = 3.1 eV. • The energy of the 7000 Ǻ photon in electron volt is 12400/7000 = 1.77 eV. • Therefore, the photon in the visible region is given in option (d). #22 • If you were asked to calculate the wave length of the photon of energy 2.48 eV, you will have • λ = 12400/2.48 = 5000 Ǻ • [You can calculate the wave length using the relation E = hc/λ where E is the energy in joule, h is Planck’s constant, c is the speed of light and λ is the wave length (in metre). In the above problem E = 2.48×1.6×10–19 joule (on converting the energy in eV into joule), c = 3×108 ms–1and h = 6.6×10–34 Js. But this is time consuming]. #23 When electromagnetic radiations of wave length λ is incident on a photosensitive surface, the kinetic energy of the photoelectrons emitted from the surface is 2 eV. When the wave length of the incident radiations is 2λ, the kinetic energy of the photoelectrons emitted from the surface is 0.5 eV. The threshold wave length (maximum wave length) for photoelectric emission from the surface is (a) λ/2 (b) λ (c) 3λ/2 (d) 2λ (e) 3λ #23 • • • • E. From Einstein’s equation, we have for the two cases hc/λ = hc/λ0 + 2 eV and hc/2λ = hc/λ0 + 0.5 eV where h is Planck’s constant, c is the speed of light and λ0 is the threshold wave length. We have expressed the kinetic energy in electron volt itself for convenience, with the understanding that all terms are in electron volt. • Multiplying the second equation by 4 and subtracting the first equation from it, we obtain • hc/λ = 3hc/λ0 from which λ0 = 3λ. #24 Heisenberg’s Uncertainty Principle states: (A) The more precise a particle’s energy can be measured, the less precise its position can be measured. (B) A particle’s position can be measured exactly. (C) A particle’s energy can be measured exactly. (D) The more precise a particle’s momentum can be measured, the less precise its position can be measured. (E) The more precise a particle’s momentum can be measured, the less precise its energy can be measured. #25 Rutherford’s Gold Foil experiment caused a modification of which of the following? (A)Plum-pudding model of the atom (B) Planetary model of the atom (C) de Broglie hypothesis (D) Wave nature of light (E) Quantum theory of light #26 In Rutherford’s Gold Foil experiment, most of the alpha particles passed through the foil undeflected. Which of the following properties of the atom can be explained from this observation? (A) The atom’s negative charge is concentrated in the nucleus. (B) The nucleus has electrons and protons. (C) The atomic mass is distributed evenly throughout the atom. (D) The alpha particles can’t be deflected by electrons. (E) The size of the nucleus is much less than the size of the atom. #27 Which of the following colors is associated with the lowest temperature of a black body radiator? (A)Violet (B) Blue (C) Green (D) Yellow (E) Red #28 Which of the following photons has the greatest energy? (A)Infrared (B) Blue light (C) X-ray (D) Gamma ray (E) Ultraviolet #29 What did Max Planck propose to solve the black body radiator problem? (A)Radiation is made up of waves. (B) Light changes its speed in different media. (C) Light comes in packets of energy. (D) Light has a continuous energy profile. (E) Objects do not radiate energy. #30 The energy of a photon depends on its: (A) Amplitude (B) Speed (C) Temperature (D) Pressure (E) Frequency #31 How does the energy of a photon change if the wavelength is doubled? (A)Doubles (B) Quadruples (C) Stays the same (D) Is cut to one-half (E) Is cut to one-fourth #32 How does the momentum of a photon change if the wavelength is halved? (A)Doubles (B) Quadruples (C) Stays the same (D) Is cut to one-half (E) Is cut to one-fourth #33 The photoelectric effect was explained by Albert Einstein by assuming that: (A)light is a wave. (B) light is a particle. (C) an electron behaves as a wave. (D) an electron behaves as a particle. (E) light does not interact with matter. #34 The kinetic energy of photoelectrons depends on the: (A)speed of light. (B) angle of illumination. (C) intensity of the light. (D) number of incident photons. (E) photon frequency. #35 The maximum kinetic energy of photoelectrons depends on which of the following? I. The light intensity II. The frequency of the light III. The material of the photoelectric cell (A) Only I (B) (B) Only II (C) (C) Only III (D) (D) Only I and II (E) (E) Only II and III #37 Classical physics could not explain the behavior of a black body radiator at very short wavelengths. What was this problem called? (A) Absorption failure (B) Ultraviolet Catastrophe (C) Wavelength catastrophe (D) Photoelectric Effect (E) Radiation