Electrical Transport and Magnetic Interactions in 3d and 5d
advertisement
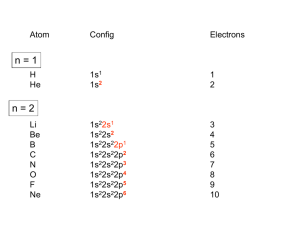
PAUL SCHERRER INSTITUT Electrical transport and magnetic interactions in 3d and 5d transition metal oxides Kazimierz Conder Laboratory for Developments and Methods, Paul Scherrer Institute, 5232 Villigen PSI, Switzerland kazimierz.conder@psi.ch Motivation For the past decades, a tremendous amount of effort has been devoted to exploring the nature of 3d transition metal oxides where various exotic states and phenomena have emerged such as: • high-Tc cuprate superconductivity • colossal magnetoresistivity • metal-insulator transitions It has been established that these states and phenomena are caused by strong cooperative interactions of spin, charge, and orbital degrees of freedom. Spin, charge, orbital and lattice degrees of freedom in strongly correlated electron systems Spin order Crystal field splitting Jahn-Teller effect Number of (unpaired) electrons: • spin • charge Lattice Spin-orbit interaction Higher cation charges: • smaller radius • smaller coord. numbers Charge order Orbital order Bond anisotropy Occupied and unoccupied orbitals 3 Electrical properties of transition metal oxides • The d-levels in most of the transition metal oxides are partially filled. • According to band structure calculations half of the known binary compounds should be conducting. Partly filled d-band Empty or completely filled d-band (d0 or d10) Orbital interaction with the lattice Octahedral crystal field Orbitals are nearby O2- Orbitals are between O2- Completely filled orbitals: d6 Energies of the d orbitals in an octahedral crystal field. http://wps.prenhall.com/wps/media/objects/3085/3159106/blb2406.html TiO- rutile Ti Ti2+ 3d24s0 O metal NiO- NaCl structure Ni2+ 3d84s0 Ni O Is insulator! Why not a metal? Why not metal? CuO Cu2+ 3d94s0 CoO Co2+ 3d74s0 MnO Mn2+ 3d54s0 Cr2O3 Cr3+ 3d34s0 Whatever is the crystal field splitting the orbitals are not fully occupied!!! Odd number of d electronsall this oxides should be metals but are insulators 3d44s2 3d54s2 Electron configurations 3d74s2 3d94s2 of elements Mott-Hubbard insulators Sir Nevill Francis Mot (on site repulsive electron force) Nobel Prize in Physics 1977 Correlation energy, Hubbard U Band width=W e- large + → + d8 + d8 → d7 + d9 Ni2+ small Ni2+ Ni3+ Ni+ U>W U<W U W Electron transfer Coulomb repulsive force Upper Hubbard band W FL FL Lower Hubbard band W Density of states U Density of states •Most of the oxides show insulating behavior, implying that the delectrons are localized. •Short-range Coulomb repulsion of electrons can prevent formation of band states, stabilizing localized electron states. 8 Mott-Hubbard insulator Charge Transfer insulator 9 Electrons have not only charge but also spin! 10 Magnetic order in transition metal oxides Diamagnetism Paramagnetism Ferromagnetism Antiferromagnetism Ferrimagnetism 11 Superexchange Superexchange is a strong (usually) antiferromagnetic coupling between two nearest neighbor cations through a non-magnetic anion. • because of the Pauli Exclusion Principle both spins on d and p hybridized orbitals have to be oriented antiparallel. • this results in antiparallel coupling with the neighbouring metal cation as electrons on porbital of oxygen are also antiparallel oriented. Fe2+ 3d6 Fe3+ 3d5 Pauli Exclusion Principle Octahedral coordination Tetrahedral coordination Magnetit (Fe3O4) inverse spinel. Ferrimagnet. Goodenough–Kanamori–Anderson Rules dx2−y2 dz 2 dz2 180o – Exchange between half occupied or empty orbitals is strong and antiferromagnetic Ferromagnetic superexchange ferromagnetic when angle 90o High Temperature Superconductor: La2-xSrxCuO4 (LaBa)2CuO4 TC=35K K.A. Müller und G. Bednorz (IBM Rüschlikon 1986, Nobel price 1987) Cu TC Metal TN Insulator 100 Antiferromagnet La, Sr Temperature [K] O La2-xSrxCuO4 Superconductor 10 0.0 0.1 0.2 0.3 Sr-content x, (holes per CuO2-layer) Undoped superconducting cuprates are antiferromagnetic Mott insulators! 14 Double-exchange mechanism Magnetic exchange that may arise between ions on different oxidation states! O2- 2p Mn 3+ d4 Mn 4+ d3 • Electron from oxygen orbital jumps to Mn 4+ cation, its vacant orbital can then be filled by an electron from Mn 3+. • Electron has moved between the neighboring metal ions, retaining its spin. • The electron movement from one cation to another is “easier” when spin direction has not to be changed (Hund's rules). La1-xCaxMnO3. Double exchange mechanism. The electron movement from one cation to another is “easier” when spin direction has not to be changed Note that no oxygen sites are shown! Ferromagnetic Metal Paramagnetic Insulator 16 CMR (colossal magnetoresistance) La0.75Ca0.25MnO3 Tc Ferromagnetic Metal Tc Magnetoresistance is defined as the relative change of resistances at different magnetic field Paramagnetic Insulator R R ( H 0) R ( H ) R(H ) A.P. Ramirez, J. Phys.: Condens. Matter., 9 (1997) 8171 17 5d vs. 3d transition metal oxides ✓ 4d and 5d orbitals are more extended than 3d’s ✓ reduced on-site Coulomb interaction strength ✓ sensitive to lattice distortion, magnetic order, etc. ✓ spin-orbit (SO) coupling much stronger • 4d and 5d orbitals are more extended than 3d’s • Reduced Coulomb interaction Insulator Metal Insulator PRB, 74 (2006) 113104 Heungsik Kim et al., Frontiers in Condensed Matter Physics, KIAS, Seoul, 2009 Sr2IrO4 Under the octahedral symmetry the 5d states are split into t52g and eg orbital states. The system would become a metal with partially filled wide t2g band. By a strong Spin-Orbit (SO) coupling the t2g band splits into effective total angular momentum Jeff=1/2 doublet and Jeff=3/2 quartet bands. Jeff = |S – L| is an effective total angular momentum defined in the t2g manifold with the spin S and the orbital angular L momenta. An unrealistically large U>> W could lead to a Mott insulator. However, a reasonable U cannot lead to an insulating state as already 4d Sr2RhO4 is a normal metal. The Jeff=1/2 spin-orbit states form a narrow band so that even small U opens a Mott gap, making it a Mott insulator The formation of the Jeff bands due to the large SO coupling energy explains why Sr2IrO4 is insulating while Sr2RhO4 is metallic. PRL 101, 076402 (2008) Interaction between the electron's spin and the magnetic field generated by the electron's orbit around the nucleus. Opposite directions of electronic orbital motions around a nucleus occur with the same probability, and thereby cancel each other. Spin and orbital motion have the same directions. The spin-orbit correlation suppresses the transfer of electrons to neighboring atoms making Sr2IrO4 an insulator. Na2IrO3 and Li2IrO3 Kitaev-Heisenberg model Crystal structure of Na2IrO3 For both Na2IrO3 and Li2IrO3 a honeycomb structure is observed enabling a realization of the exactly solvable spin model with spin liquid ground state proposed by Kitaev. monoclinic space group C 2/m Iridium honeycomb layers stacked along the monoclinic c axis PRB 88, 035107 (2013) 22 Na2IrO3 and Li2IrO3 Kitaev-Heisenberg model Kitaev exchange Heisenberg exchange J>0 ferromagnetic J<0 antiferromagnetic A Spin Liquid (Figure Credits: Francis Pratt, STFC) J1=0 J1=2J2 J2=0 PRL 105, 027204 (2010) 23 Na2IrO3 and Li2IrO3 Kitaev-Heisenberg model A Spin Liquid (Figure Credits: Francis Pratt, STFC) • Na2IrO3 and Li2IrO3 order magnetically at 15K • I was suggested (PRB 84, 100406 (2011)) that the reduction of the chemical pressure along the caxis can induce spin glass behavior. • This can be achieved either by exerting pressure in the ab plane or substituting Na by smaller Li ions. 24 Na2-xLixIrO3 with x = 0, 0.05, 0.1 and 0.15 For higher doping spin-glass state Na2IrO3 Na1.95Li0.05IrO3 Glassy state The cusp is frequency dependent which is characteristic for the spin-glass phase • Antiferromagnetic transition around 15K for the parent compound Na2IrO3. • This is suppressed for the doped sample. Magnetization measurements of Na1.9Li0.1IrO3 in 0.1T. Real and imaginary part of the AC susceptibility measured at different frequencies. K. Rolfs, S. Toth, E. Pomjakushina, D. Sheptyakov, K. Conder, to be published Conclusions 5d iridates: • crystal field splitting • spin-orbit interaction Electrical transport properties in transition metals (Mott insulators): • crystal field splitting • Coulomb repulsion Colossal magnetoresistivity: • crystal field splitting • orbital order 26 27