Chapter 9
advertisement
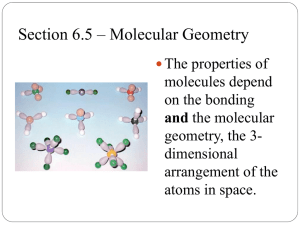
Chapter 9 Molecular Geometry: Shape Determines Function Chapter Outline • 9.1 Molecular Shape • 9.2 Valence-Shell Electron-Pair Repulsion Theory (VSEPR) • 9.3 Polar Bonds and Polar Molecules • 9.4 Valence Bond Theory • 9.5 Shape and Interactions with Large Molecules • 9.6 Chirality and Molecular Recognition • 9.7 Molecular Orbital Theory © 2014 W. W. Norton Co., Inc. 9-2 Molecular Shape • Chemical/physical properties are related to molecular shape. • Lewis structures • Show atoms and bonds, but not spatial orientations (3D). • Molecular models • Show orientations and bond angles; help us understand physicochemical properties. © 2014 W. W. Norton Co., Inc. 9-3 Lewis Structures vs. Models © 2014 W. W. Norton Co., Inc. 9-4 Molecular Shape (cont.) • Bond angle: • Angle (in degrees) defined by lines joining the centers of two atoms to the center of a third atom to which they are covalently bonded • Not always predictable from Lewis structures © 2014 W. W. Norton Co., Inc. 9-5 Chapter Outline • 9.1 Molecular Shape • 9.2 Valence-Shell Electron-Pair Repulsion Theory (VSEPR) • Central Atoms with No Lone Pairs • Central Atoms with Lone Pairs • 9.3 Polar Bonds and Polar Molecules • 9.4 Valence Bond Theory • 9.5 Shape and Interactions with Large Molecules • 9.6 Chirality and Molecular Recognition • 9.7 Molecular Orbital Theory © 2014 W. W. Norton Co., Inc. 9-6 Valence-Shell Electron-Pair Repulsion Theory (VSEPR) • VSEPR Theory: • A model predicting that the arrangement of valence electron pairs around a central atom minimizes repulsion to produce the lowest-energy orientation. • Electron-pair geometry: • Three-dimensional arrangement of bonding e– pairs and lone pairs electrons about a central atom • Molecular geometry: • 3-dimensional arrangement of atoms in a molecule. © 2014 W. W. Norton Co., Inc. 9-7 VSEPR: Electron-Pair Geometry • To determine electron-pair geometry: • Draw Lewis structure (see Chapter 8). • From Lewis structure, determine steric number (SN): • • Determine optimal spatial arrangement of electron pairs (bonding + nonbonding) to minimize repulsion. © 2014 W. W. Norton Co., Inc. 9-8 Molecular Geometry: Central Atom with No Lone Pairs • Molecular geometry = electron-pair geometry • Determine steric number (SN): • SN = 2 (two atoms bonded to central atom) • geometry linear • SN = 3 (three atoms bonded to central atom) • geometry trigonal planar • SN = 4 tetrahedral • SN = 5 trigonal bipyramidal • SN = 6 octahedral © 2014 W. W. Norton Co., Inc. 9-9 Central Atoms with No Lone Pairs © 2014 W. W. Norton Co., Inc. 9 - 10 Central Atoms with No Lone Pairs (cont.) © 2014 W. W. Norton Co., Inc. 9 - 11 Geometric Forms • Examples: • CO2 BF3 © 2014 W. W. Norton Co., Inc. CCl4 PF5 SF6 9 - 12 Practice: Molecular Geometry (No Lone Pairs) • Determine the molecular geometry of: a) H2CO (C is central atom) • b) CH4 - Collect and Organize: We are given molecular formulas and asked to predict their molecular geometry. © 2014 W. W. Norton Co., Inc. 9 - 13 Practice: Molecular Geometry (No Lone Pairs) • Determine the molecular geometry of: a) H2CO (C is central atom) • b) CH4 - Analyze: We can use the periodic table to determine the number of valence electrons for each atom. From the molecular formula and valence electrons, we can draw Lewis structures. From the Lewis structures we can determine the SN. From the SN we can predict the electronpair geometry. Since there are no lone pairs, the electronpair geometry is the same as the molecular geometry. © 2014 W. W. Norton Co., Inc. 9 - 14 Practice: Molecular Geometry (No Lone Pairs) • Determine the molecular geometry of: a) H2CO (C is central atom) • b) CH4 -Solve: H2CO C is the central atom, with single bonds to each H atom and a double bond to O, SN = 3. Molecular geometry = trigonal planar. CH4 C is central atom, with single bonds to each H atom, SN = 4. Molecular geometry = tetrahedral. © 2014 W. W. Norton Co., Inc. 9 - 15 Practice: Molecular Geometry (No Lone Pairs) • Determine the molecular geometry of: a) H2CO (C is central atom) • b) CH4 - Think About It: The molecular geometries are consistent with VSEPR theory for 3 and 4 electron clouds. It is worth noting that the C atom always has 4 bonds, but a double bond counts as only one electron cloud, resulting in a trigonal planar geometry. © 2014 W. W. Norton Co., Inc. 9 - 16 Central Atoms with Lone Pairs • Molecular geometry electron-pair geometry • Replace bonding pair(s) with lone pair(s). • Example: SO2 (SN = 3) • Three electron pairs (2 bonding + 1 lone pair) © 2014 W. W. Norton Co., Inc. 9 - 17 Central Atoms w/ Lone Pairs (cont.) • Bond angles less than predicted • Electron pair repulsion! • Lone pair–lone pair = greatest repulsion. • Lone pair–bonding pair • Bonding pair–bonding pair = least repulsion. • Multiple bonds > single bonds © 2014 W. W. Norton Co., Inc. 9 - 18 Molecular Geometry: SN = 4 Note: bond angles decrease as # of lone pairs increases. © 2014 W. W. Norton Co., Inc. 9 - 19 Molecular Geometry: SN = 4 (cont.) Two lone pairs = greater repulsion, decreased bond angle. © 2014 W. W. Norton Co., Inc. 9 - 20 Molecular Geometry: SN = 5 Note: lone pairs occupy equatorial positions. © 2014 W. W. Norton Co., Inc. 9 -21 Molecular Geometry: SN = 6 Note: bond angles = 90 (geometries w/ more than 2 lone pairs are possible.) © 2014 W. W. Norton Co., Inc. 9 - 22 Practice: Molecular Geometry • What are the molecular geometries of the ions: SCN– and NO2– ? - Collect and Organize: We are given the molecular formulas for two polyatomic ions and asked to predict the molecular geometries. © 2014 W. W. Norton Co., Inc. 9 - 23 Practice: Molecular Geometry • What are the molecular geometries of the ions: SCN– and NO2– ? -Analyze: We can use the periodic table to determine the number of valence electrons for each atom. From the molecular formula and valence electrons, we can draw Lewis structures. From the Lewis structures, we can determine the SN. From the SN, we can predict the electron-pair geometry. Making note of the number of bonding pairs and lone pairs, we can identify the molecular geometry. © 2014 W. W. Norton Co., Inc. 9 - 24 Practice: Molecular Geometry • What are the molecular geometries of the ions: SCN– and NO2– ? Solve: SCN– As the least electronegative element and the one with the greatest bonding capacity, C is the central atom. Although there are several possible resonance structures, they all have C with SN = 2 and no lone pairs. Molecular geometry = linear. NO2– With N as the central atom, the Lewis structure has N with SN = 3 (two bonding pairs and one lone pair). Again, although there are two possible resonance structures, they both have the same SN value. Molecular geometry = bent, with bond angle <120 due to the extra repulsive energy of the lone pair on N. © 2014 W. W. Norton Co., Inc. 9 - 25 Practice: Molecular Geometry • What are the molecular geometries of the ions: SCN– and NO2– ? -Think About It: It is worth noting that both molecular structures have two bonding electron clouds, but different molecular geometries due to the differences in steric number. © 2014 W. W. Norton Co., Inc. 9 - 26 Chapter Outline • 9.1 Molecular Shape • 9.2 Valence-Shell Electron-Pair Repulsion Theory (VSEPR) • 9.3 Polar Bonds and Polar Molecules • What Makes a Molecule Polar? • Dipole Moments • 9.4 Valence Bond Theory • 9.5 Shape and Interactions with Large Molecules • 9.6 Chirality and Molecular Recognition • 9.7 Molecular Orbital Theory © 2014 W. W. Norton Co., Inc. 9 - 27 Polar Bonds and Polar Molecules • Requirements for polar molecule: • 1. Polar bonds (i.e. covalent bond between atoms with ΔEN). • 2. Nonuniform distribution of polar bonds. © 2014 W. W. Norton Co., Inc. 9 - 28 Polar Molecules (cont.) • Bond dipole: • Separation of electrical charge created when atoms with different EN form a covalent bond. • Polar molecule: • Vectors of bond dipoles whose sum > zero. © 2014 W. W. Norton Co., Inc. 9 -29 Measuring Polarity • Dipole moment () – a quantitative expression of the polarity of a molecule. • Units = debyes (D); 1 D = 3.34 × 10–30 C·m ) © 2014 W. W. Norton Co., Inc. 9 - 30 © 2014 W. W. Norton Co., Inc. 9 -31 Chapter Outline • 9.1 Molecular Shape • 9.2 Valence-Shell Electron-Pair Repulsion Theory (VSEPR) • 9.3 Polar Bonds and Polar Molecules • 9.4 Valence Bond Theory • Bonds from Orbital Overlap • Hybridization (sp3, sp2, sp, sp3d, sp3d2) • 9.5 Shape and Interactions with Large Molecules • 9.6 Chirality and Molecular Recognition • 9.7 Molecular Orbital Theory © 2014 W. W. Norton Co., Inc. 9 - 32 Atomic Orbitals and Bonds • • Valence Bond Theory (Linus Pauling) • Quantum mechanics-based model • Covalent bond = overlap of half-filled orbitals Sigma () bond: • Covalent bond in which the highest electron density lies between the two atoms along the bond axis. Overlap of 1s orbitals © 2014 W. W. Norton Co., Inc. 9 - 33 Hybridization: sp3 Orbitals • Hybridization – the mixing of atomic orbitals to generate new sets of orbitals that form covalent bonds with other atoms. © 2014 W. W. Norton Co., Inc. 9 - 34 Tetrahedral Sigma Bonds • Tetrahedral orientation of sp3 hybridized orbitals = tetrahedral molecular geometry Overlap of 1s with sp3 orbitals © 2014 W. W. Norton Co., Inc. 9 - 35 Other sp3 Hybrid Examples Note: lone pairs (nonbonding) © 2014 W. W. Norton Co., Inc. 9 - 36 Trigonal Planar: sp2 Hybridization Unhybridized p orbitals form double bonds. © 2014 W. W. Norton Co., Inc. 9 - 37 sp2 Hybridization (cont.) • pi () bond: a covalent bond in which electron density is greatest around—not along—the bonding axis. © 2014 W. W. Norton Co., Inc. 9 - 38 Linear: sp Hybridization Form triple bond (one and two π bonds). © 2014 W. W. Norton Co., Inc. 9 - 39 Trigonal Bipyramidal: sp3d Hybridization Formed by mixing one s, one d, and three p orbitals. Example: PF5 – five sigma bonds © 2014 W. W. Norton Co., Inc. 9 - 40 Octahedral: sp3d2 Hybridization Formed by mixing one s, two d, and three p orbitals. Example: SF6 – six sigma bonds © 2014 W. W. Norton Co., Inc. 9 - 41 © 2014 W. W. Norton Co., Inc. 9 -42 Practice: Hybrid Orbitals What are the hybridizations of the central atoms of the ions: SCN– and NO2– ? - Collect and Organize: Note that these are the same molecules for which we determined molecular geometry back in section 9.2. Using Lewis structures and VSEPR, we determined that the electronic geometry around the central carbon in SCN– was linear (SN = 2), and the electronic geometry around the central N atom in NO2– was trigonal planar (SN = 3). © 2014 W. W. Norton Co., Inc. 9 - 43 Practice: Hybrid Orbitals What are the hybridizations of the central atoms of the ions: SCN– and NO2– ? - Analyze: From the steric number of the central atoms and valence bond theory, we can determine the hybridization around the central atom based on electron-pair geometry. © 2014 W. W. Norton Co., Inc. 9 - 44 Practice: Hybrid Orbitals What are the hybridizations of the central atoms of the ions: SCN– and NO2– ? Solve: SCN– is linear (SN = 2), so the hybridization must be sp. NO2– is trigonal planar (SN=3), so the hybridization must be sp2. © 2014 W. W. Norton Co., Inc. 9 - 45 Chapter Outline • 9.1 Molecular Shape • 9.2 Valence-Shell Electron-Pair Repulsion Theory (VSEPR) • 9.3 Polar Bonds and Polar Molecules • 9.4 Valence Bond Theory • 9.5 Shape and Interactions with Large Molecules • Shape and Molecular Recognition • Delocalized Electrons • 9.6 Chirality and Molecular Recognition • 9.7 Molecular Orbital Theory © 2014 W. W. Norton Co., Inc. 9 - 46 Molecular Recognition • Molecular recognition: • The process by which molecules interact with other molecules in living tissues to produce a biological effect. • Example: ethylene (ripening agent) All atoms in same plane. © 2014 W. W. Norton Co., Inc. 9 - 47 Delocalization of Electrons • Delocalization: spreading of electrons in alternating single and double bonds over three or more atoms in a molecule a) b) © 2014 W. W. Norton Co., Inc. 9 -48 Aromatic Compounds • Aromatic compound: • A cyclic, planar compound with delocalized electrons above and below the plane of the molecule. • Example: polycyclic aromatic hydrocarbons (PAH) • Planar shape may allow intercalation in DNA © 2014 W. W. Norton Co., Inc. 9 - 49 Chapter Outline • 9.1 Molecular Shape • 9.2 Valence-Shell Electron-Pair Repulsion Theory (VSEPR) • 9.3 Polar Bonds and Polar Molecules • 9.4 Valence Bond Theory • 9.5 Shape and Interactions with Large Molecules • 9.6 Chirality and Molecular Recognition • Optical Isomerism • 9.7 Molecular Orbital Theory © 2014 W. W. Norton Co., Inc. 9 - 50 Isomerism • Isomers – compounds with same molecular formula but with atoms arranged differently in three-dimensional space. Optical isomers © 2014 W. W. Norton Co., Inc. 9 - 51 Optical Isomers = Chirality • Chirality – property of a molecule that is not superimposable on its mirror image. © 2014 W. W. Norton Co., Inc. 9 - 52 Chapter Outline • 9.1 Molecular Shape • 9.2 Valence-Shell Electron-Pair Repulsion Theory (VSEPR) • 9.3 Polar Bonds and Polar Molecules • 9.4 Valence Bond Theory • 9.5 Shape and Interactions with Large Molecules • 9.6 Chirality and Molecular Recognition • 9.7 Molecular Orbital Theory • • • • Molecular Orbitals of Hydrogen and Helium MOs of Homonuclear Diatomic Molecules MOs of Heteronuclear Diatomic Molecules MOs of N2+ and Spectra of Auroras © 2014 W. W. Norton Co., Inc. 9 - 53 Molecular Orbital (MO) Theory • Based on mixing of atomic orbitals of similar shapes and energies to form molecular orbitals (MOs) that belong to the molecule as a whole • The number of MOs formed is equal to the number of atomic orbitals combined. • MOs represent discrete energy states; orbitals spread out over entire molecule. © 2014 W. W. Norton Co., Inc. 9 - 54 Types of Molecular Orbitals • Bonding orbitals: • Region of increased electron density between nuclear centers that hold atoms together • Are lower in energy (more stable) than atomic orbitals from which they are formed • Antibonding orbitals: • Region of electron density that destabilize the molecule because they do not increase electron density between nuclear centers • Less stable than atomic orbitals from which they are formed © 2014 W. W. Norton Co., Inc. 9 - 55 Molecular Orbital Diagrams • MO diagram: • Energy-level diagram for molecular showing the relative energies and electron occupancy of the MOs for a molecule. • Sigma (σ) bond: • Covalent bond with the highest electron density along the bond axis. • Pi (π) bond: • Bond formed by mixing of atomic orbitals not oriented along the bonding axis in a molecule. © 2014 W. W. Norton Co., Inc. 9 - 56 Molecular Orbital Diagram: H2 The two 1s orbitals mix to yield two sigma MOs (1 bonding/1 antibonding). © 2014 W. W. Norton Co., Inc. 9 -57 Bond Order and Stability Bond order = 1/2 (# bonding e– – # antibonding e–) Bond order in H2 – = ½ (Stable) © 2014 W. W. Norton Co., Inc. Bond order in He2 = 0 (Not stable) 9 - 58 MO Guidelines 1. The total # of MOs = the # of AOs orbitals mixed. 2. Orbitals with similar energy/shape mix more effectively than do those of different energy/shape. 3. Orbitals of different n (different sizes/energies) result in less effective mixing. 4. A MO can accommodate two electrons. 5. Electrons fill MO diagrams according to Hund’s rule. © 2014 W. W. Norton Co., Inc. 9 - 59 MO Scheme for O2 • Electron configuration for O2: (σ2s)2(σ2s*)2(σ2p)2(π2p)4(π2p *)2 • Bond order = ½ (8 – 4) = 2 • O2 has two bonds • O2 has two unpaired electrons in π2p* © 2014 W. W. Norton Co., Inc. 9 - 60 MO Scheme for N2 • Electron configuration for N2: (σ2s)2(σ2s*)2(σ2p)2(π2p)4 • Bond order = ½ (8 – 2) =3 N2 has three bonds. N2 has no unpaired electrons. © 2014 W. W. Norton Co., Inc. 9 - 61 Paramagnetic vs. Diamagnetic • Paramagnetism: • Atoms or molecules having unpaired electrons are attracted to magnetic fields. • Example: O2 • Diamagnetism: • Atoms or molecules having all paired electrons are repelled by magnetic fields. • Example: N2 © 2014 W. W. Norton Co., Inc. 9 - 62 MO for NO • Zeff alters MO diagram; atomic orbitals for O are lower in energy. • Odd electron in π*2p , closer in energy to the 2p atomic orbitals of N atom. • Bond order = ½ (8 – 3) = 2.5 © 2014 W. W. Norton Co., Inc. 9 - 63 MO for N2, N2+: Emission Spectra Crimson red © 2014 W. W. Norton Co., Inc. Blue-violet 9 - 64 ChemTours: Chapter 9 Click here to launch the ChemTours website © 2014 W. W. Norton Co., Inc. 9 - 65