Arik Levy - Tumor Detection Using Antibodies Conjugated Magnetic
advertisement
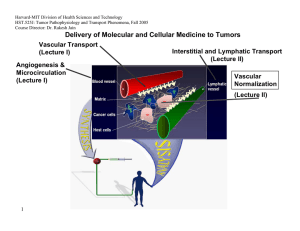
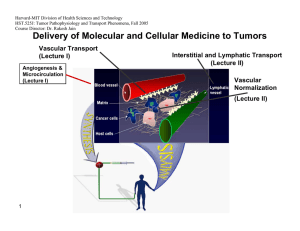
Tumor detection using antibodies conjugated magnetic nanoparticles Arie Levy, Israel Gannot Biomedical Engineering Department Tel Aviv University Israel Thermography First Introduced at 1956 [1] Increased angiogenesis and metabolism around tumors [2] Temperature rise at the skin surface above the tumor. Detection by IR cameras. Computer aided detection Thermography - cont Advantages: [2] Radiation free Contact free Non Invasive Low Cost Disadvantages Low sensitivity for small & deep tumors [3] Not tumor specific Subjective Detect & Treat Approach 2. Tumor Detection 1. MNP Injection Tumor + accumulated MNP Tumor IR Camera, used as a detector Antibody conjugated MNP solution 3. Treatment Low power external AMF IR camera used as a sensor for feedback High power external AMF TBT vs. Thermography The heat can be turned on and off – good reference can be achieved The heat emanating from the tumor is considerably larger. The heat source is tumor specific. Objective- no need for special skills. Treatment can be combined at the same session. Magnetic [4] Nanoparticles Magnetic Nanoparticles Magnetic Nanoparticles Coil Magnetic Nanoparticles Targeting Small enough to diffuse from blood vessel Antibodies targeting Binding sites HER2 – Breast Cancer[5]. MN – renal cell carcinoma [6] U251-SP (G22 antibody) – Glioma [7] Antibody Coating Magnetic Nanoparticle Experiment Setup DC power supply DAQ unit 0.5mm polymeric cover 70x40mm glass cup filled with US Gel 1KΩ SMT resistor Micrometer stage Experiment Setup – cont. IR Camera RF Generator Coil Tissue Phantom Tumor Phantom Problem Definition & Assumptions Small tumor (<5mm) – point heat source. The tissue was numerically modeled using COMSOL according the Pennes bioheat equation [8]: Thermal properties – conductivity , perfusion . metabolism – are assumed. Unknown Location (X,Y, Depth). D 2mm Tumor Tissue Tissue Surface Tumor Detection Challenge The temperature difference at the tissue surface is very low regarding measurement noise level Without Noise With Noise Detection Protocol 1. 2. 3. 4. Reference data is recorded. Magnetic field/heat source is turned on. Sequence of IR images is recorded. The data is processed using MATLAB in order to detect the tumor and its location. Detection Algorithm Pre Processing Input Noise Filtering data set Hot Spot Detection Hot Spot Classification Time Averaging Reference data set Pre calculated estimation Tumor size & location Pre Processing Original IR Data Filtered Data Original Data Minus Reference Data Region of Interest Selection Hot Spot Selection “True” Hot Spot Temperature change [Deg C] “False” Hot Spot ROI border Hot Spot Classification Temperature change [Deg C] 2mm Hot Spot Hot Spot Classification Temperature change [Deg C] 12mm Hot Spot Hot spot classification Normalization of each prediction to the hot spot data. Calculating matching value for each prediction: 2 w ( d p ) k k i j i , j , k i , j , k Mv 1 2 k wk i j (di, j ,k ) Thresholding. Interpolation. Depth estimation according to maximum matching. Hot Spot Classification Best match: 4mm prediction Normalized predicted temperature change for tumor depths 1-10[mm] Recorded Temperature change Hot Spot Classification Detection Threshold Max at 4mm Prediction Depth [mm] Experiments Setup 1 (US gel): 3 different emitted powers. Up to 14mm depth. Idle (“no tumor”) measurement. Setup 2 (Procine). Validation using 3mm depth tumor. Training 140 measurements for idle (“no tumor”) and worst case (13mm 400mW) states. Idle and worst case stats distribution of peak value Tumor set Tumor gaussian fit Idle set Idle gaussian fit 0.25 0.2 Probability 0.15 0.1 0.05 0 0 0.001 0.002 0.003 0.004 0.005 0.006 Peak value [K] 0.007 0.008 0.009 0.01 Sensitivity & Specificity Specificity:98.68% Depth Estimation Other Results Low power detection. Procine model validation. Magnetic Acoustic Detection -MAD Acoustic sensor Magnetic coil Pulsed magnetic field Magnetically marked tumor Tissue Acoustic shock wave MAD - Simulation MAD – Experimental Setup MAD - Results Summary TBT Up to 14mm detection was demonstrated. Sub-millimeter tumors can be detected. Highly specific detection. Limited to near to surface tumors. MAD Potentially could detect deeper tumors. Simple setup. Future work TBT: Algorithm refinement. In vivo validation. MAD Proof of concept. Setup improvement. Treatment. Rotating Magnetic field. Double conjugation. Thank You… Reference 1. R. N. Lawson. Implications of surface temperature in the diagnosis of breast cancer. Canada Med Assoc J, 75:309– 310, 1956 2. WC Amalu. Infrared imaging of the breast – an overview. Medical device and systems, cahpter 25, 2006 3. Statement on use thermography to detect breast cancer, NBCC, 1999, www.nbcc.org.au. 4. Kalambur V S, Han B, Hammer B E, Shield T W and Bischof J C 2005 In vitro characterization of movement,heating and visualization of magnetic nanoparticles for biomedical applications Nanotechnology 16 1221–33 Reference 5. Akira Ito et al. Magnetite nanoparticle-loaded anti-HER2 immunoliposomes, for combination of antibody therapy with hyperthermia, Cancer Letters 212 (2004) 167–175 6. M Shinkai et al. Targeting Hyperthermia for Renal Cell Carcinoma Using Human MN Antigenspecific Magnetoliposomes. Jpn. J. Cancer Res. 92, 1138–1146, 2001 7. Biao LE et al , Preparation of tumor-specific magnetoliposomes and their application for hyperthermia, Chem. Eng. Jpn, 2001 8. HH Pennes. Analysis of Tissue and Arterial Blood Temperatures in the Resting Human Forearm. Journal of Applied Physiology, 1948