Principle of Spectroscopy
advertisement

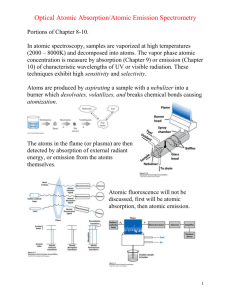
Atomic Absorption Spectroscopy (AAS)I Mentor : Prof. Kuniyuki KITAGAWA Assistant: Dr. Eng.Nelfa Desmira Visible and Ultraviolet Light Uv-Vis Spectrometer Double Beam Light Source UV (Deuterium/D2) 1a 2 Mirror 1 Slit 1 Slit 2 1b Light Source Vis (Tungsten) 3 Detector-2 Filter Reference Beam 6a Reference Cuvette 7a I Lens 1 Half Mirror 5 4 Detector-1 6b Sample Beam Sample Cuvette 7b Lens 2 I 0 Uv-Vis Spectrometer Double Beam • • • • • • • • No 1a and 1 b – The UV-Visible spectrophotometer uses two light sources, a deuterium (D2) lamp for ultraviolet light and a tungsten (W) lamp for visible light. Two light sources hit Mirror 1 one pass through slit 1 go to diffraction grating (no.2) No 2 – The grating is able to rotated so a specific wavelength is selectable. From diffraction grating goes to slit 2 and filter (No. 3) No 3 – A filter is used to remove unwanted higher orders of diffraction. No 4 – The light beam hits a second mirror No 5 – splited by a half mirror to 6a and 6b No 6a and 6b – Half of the light is reflected (6a), the other half passes through (6b) No 7a – One of the beams is allowed to pass through a reference cuvette. No 7b – the other passes through the sample cuvette. The intensities of the light beams are then measured at the end Beer-Lambert Law • The Beer-Lambert law is the linear relationship between absorbance and concentration of an absorbing species. • Experimental measurements are usually made in terms of transmittance (T), which is defined as: T = I / Io where I is the light intensity after it passes through the sample and Io is the initial light intensity. • The relation between A and T is: A = -log T = - log (I / Io) where A is the measured absorbance Transmittance The relationship between absorbance and transmittance is illustrated in the following diagram: Assignment • Calculate the transmission for absorbance A 0.6 and 0.06 • How to make correction for background absorption caused by sample matrices using a one-beam spectrophotometer? • Explain the system of single beam Uv-Vis Spectrometer Atomic Absorption Spectroscopy (AAS) II Mentor : Prof. Kuniyuki Kitagawa Assistant : Dr. Eng. Nelfa Desmira Atomic Spectroscopy • A method to analyze the elemental composition using atomic absorption or emission • Energy transition electrons of atoms E1 E2 E3 Ground State E0 Emission Excited State Excited State Absorption E1 E2 E3 Ground State h = Ei– E0 h = Ej – Ei i = 1.2 and 3 i = 1.2 and 3 Ej v1 1 v2 2 v3 3 1 2 3 Atomic Absorption Spectroscopy Ej I0 E0 Beer Lambert Law : I Where : A = absorbance/Emission T = Transmitance Atomic Absorption Spectroscopy : The term used when the radiation absorbed by atoms is measured E0 and Ej : energy levels where Ej higher than E0. Arrow line: Absorption Atomic Absorption Spectrometry Radiation Source Lens Atomized Sample (Flame) Lens Monochromator Detector Output Flame Atomic Absorption Spectrometry AAS consist of two type: Flame AAS and Graphite-furnace AAS. Please open this link : http://www.cee.vt.edu/ewr/environmental/teach/smprimer/aa/aadiag.gif Atomic Absorption Spectrometry • Radiation Source – Hollow-Cathode Lamp • Atomization Sample – AAS analyzes atoms in gas phase so atoms in a sample must heated/vaporized in a hightemperature source such as a flame or graphite furnace. Flame AA is suitable to analyze solutions, while graphite furnace AA is able to analyze solutions, slurries, or solid samples. Atomic Absorption Spectrometry • Monochromator and Detector – AA spectrometers use monochromators and detectors for UV and visible light. – Monochromator is used to isolate the absorption line from background light due to interferences. Assignment • Explain the detail of flame atomization absorption spectrometry (FAAS) • Explain the detail of graphite furnace atomization absorption spectrometry (GAAS) • Compare the detection limits of FAAS and GAAS and mention their applications respectively