X-ray fluorescence (XRF)
advertisement
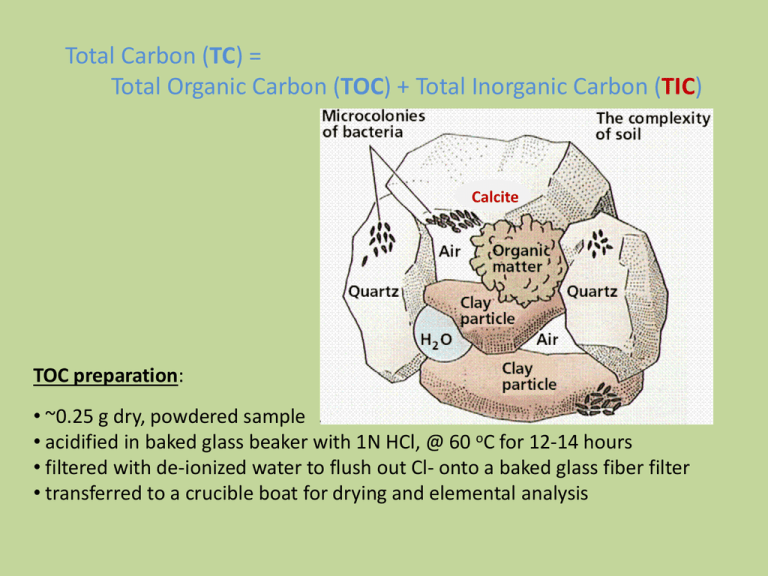
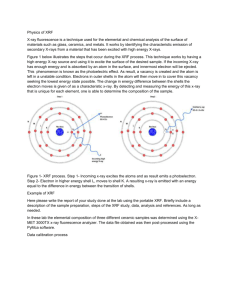
Total Carbon (TC) = Total Organic Carbon (TOC) + Total Inorganic Carbon (TIC) Calcite TOC preparation: • ~0.25 g dry, powdered sample • acidified in baked glass beaker with 1N HCl, @ 60 oC for 12-14 hours • filtered with de-ionized water to flush out Cl- onto a baked glass fiber filter • transferred to a crucible boat for drying and elemental analysis Trace elements coprecipitated with secondary soil minerals and soil organic matter (SOM) Solid Fe and Al oxides Mn oxides Ca carbonates Illites Smectites Vermiculites Organic matter Coprecipitated trace elements B, P, V, Mn, Ni, Cu, Zn, Mo, As, Se P, Fe, Co, Ni, Zn, Mo, As, Se, Pb P, V, Mn, Fe, Co, Cd B, V, Ni, Co, Cr, Cu, Zn, Mo, As, Se, Pb B, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Pb Ti, Mn, Fe Al, V, Cr, Mn, Fe, Ni, Cu, Zn, Cd, Pb, P, N Carmichael Creek, MT P2O5 (wt. %) 0.3 CCN south-facing 0.25 0.2 4A 0.15 3A 4A 1A CCS north-facing 1A 2A 3A 2A 0.1 0.05 0 0 1 2 3 4 Total Organic Carbon (wt%) 5 X-ray fluorescence (XRF) The technology was developed in the 1950’s. X-ray fluorescence (XRF): The emission of characteristic "secondary", or fluorescent, X-rays from a material that has been excited by bombarding with highenergy X-rays or gamma rays. www.niton.com/.../primary-x-ray-radiation.jpg Irradiating an atom with high-energy primary X-ray photons delivers sufficient energy for an electron to be ejected completely out of the atom. An outer shell L electron falls inward to fill the void created in the inner shell, and an X-ray characteristic of the atom's elements is emitted. X-ray fluorescence (XRF) spectrometers Bragg’s Law nλ= 2d * sinΘ Schematic arrangement of wavelength dispersive spectrometer. Schematic arrangement of energy dispersive spectrometer The dispersion and detection are a single operation. Proportional counters or various types of solid state detectors (PIN diode, Si(Li), Ge(Li), Silicon Drift Detector SDD) are used. http://en.wikipedia.org/wiki/X-ray_fluorescence Schematic arrangement of a wavelength-dispersive (WD) X-ray fluorescence (XRF) spectrometer A. B. Various types of detectors most commonly gas flow proportional and scintillation are used to measure the intensity of the emitted beam on bench top models. A. Gas flow proportional counters are used mainly for detection of longer wavelengths (lighter elements). The gas is usually 90% argon, 10% methane ("P10"). The argon is ionized by incoming X-ray photons, and the electric field multiplies this charge into a measurable pulse. The methane suppresses the formation of fluorescent photons caused by recombination of the argon ions with stray electrons. B. Scintillation counters consist of a scintillating crystal (typically of sodium iodide doped with thallium) attached to a photomultiplier. The crystal must be protected with a relatively thick aluminum/beryllium foil window, which limits the use of the detector to wavelengths below 0.25 nm. Typically used for heavier elements. Scintillation - a flash of light produced in certain materials when they absorb ionizing radiation. Pressed Powder Disks/Pellets To obtain good XRF results using the pressed powder technique, control of particle size is absolutely critical. Used primarily for trace elements and uniform samples compositions. Fused Glass Disk For Major Oxide Analysis Lithium metaborate flux + sample in a 7:1 proportion Fusion in Pt crucible > 750 oC Fused glass disk from gold or brass mould Sample Preparation Typical Composition of Grinding Units Material Hardened Steel Stainless Steel Cr-free Steel Tungsten Carbide Alumina Ceramic Major Elements Minor Elements Hardness Resistance to (Mohs) Abrasion Fe Cr, Si, Mn, C 5.5-6 Moderate High Fe, Cr Ni, Mn, S, Si 5-5.5 Moderate High Fe C, Mn, Si, Mo 5-5.5 Moderate High W, C, Co Ta, Ti, Nb 8.5 + High Long-wearing, but brittle Al 9 Long-wearing, but brittle 8.5 Agate Si Si, Ca, Mg Al, Na, Fe, K, Ca, Mg Zirconia Silicon Nitride Zr Hf, Mg 8.5 Si Y, Al, Fe, Ca 8.5 + Very High Extremely High Extremely High Extremely High Plastic C -- 1.5 Low Available in the Department Durability Very long-wearing, but brittle Very long-wearing Very long-wearing Low, but disposable Trace Elements Detection limit 2 ppm: Zr Pb Sr Ni Rb Y Cr Zn Nb Co Detection limit 5 ppm: Cu W Mo Th Detection limit 10 ppm: U V Detection limit 50 ppm: Ba Major Oxides Detection limit 0.02 %: Al2O3 Fe2O3 P2O5 MnO TiO2 Loss On Ignition (LOI) is carried out at 1000oC. Removes volatiles including carbon, sulfur and nitrogen compounds, and structural and adsorbed water (H2O). Detection limit 0.02 %: LOI wt.% = (sample weight - residue weight) * 100 sample weight CaO K2O Na2O MgO SiO2 Laboratory Bench-top Models Field Hand-held Models Linear Regressions for Select Trace Elements using Standard Reference Materials (SRMs) 160 140 120 100 80 60 40 20 0 Nb Regression 300 250 Nb (ppm) Mo (ppm) Mo Regression y = 28.399x R2 = 0.9894 200 150 y = 17.083x R2 = 0.9959 100 50 0 0 1 2 3 4 5 0 5 intensity Mo 200 400 intensity Sr Zr (ppm) Sr (ppm) 20 Zr Regression y = 0.0399x R2 = 0.9348 0 15 intensity Nb Sr Regression 35 30 25 20 15 10 5 0 10 600 800 1400 1200 1000 800 600 400 200 0 y = 16.189x R2 = 0.9875 0 20 40 intensity Zr 60 80 PD Soil Profile Major Oxide Concentrations (wt.%) Core #1 Trace Elements Concentrations (ppm) Profile Horizons Al2O3 A Fe2O3 AB Na2O MgO Bt Bxta Bxtb II Bt Zr II Bx Sr II Bx2 V 0 5 10 15 20 0 100 200 300 400 500 PD Soil Profile Major Oxide Concentrations (wt.%) Core #1 Trace Elements Concentrations (ppm) Profile Horizons Al2O3 A Fe2O3 AB Na2O MgO Bt Bxta Bxtb II Bt Zr II Bx Sr II Bx2 V 0 5 10 15 20 0 100 200 300 400 500