Chapter 5 Enzymes, Coenzyme and Energy
advertisement
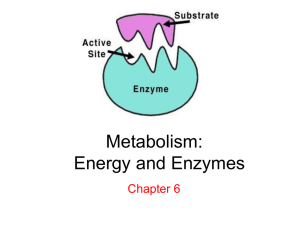
Chapter 5 Enzymes, Coenzyme and Energy Biology 100 Spring 2009 Energy All living things require energy. ◦ Nutrients are one source of energy, as well as being molecules organisms require to grow, reproduce or repair Biochemical reactions are the processes used for the formation, breakdown and rearrangement of molecules to provide organisms with energy Activation Energy Activation Energy is the required input of energy to make a reaction start Catalyst A catalyst is a chemical that speeds up the reaction but is not used up in the reaction ◦ Lowers the activation energy needed to start a reaction ◦ Is not used up during the reaction ◦ Is unchanged after a reaction Enzymes Enzymes act as catalysts. Enzymes are proteins that speed up a rate of reaction ◦ ◦ ◦ ◦ Found in cells throughout the body Lowers activation energy Enzymes will end in –ase SPECIFIC! Enzymes How Enzymes Work Each enzyme has a specific size and 3-D shape ◦ Each enzyme is going to fit with a certain substrate (molecule enzyme connects to) How Enzymes Work When the enzyme and substrate are connected, it is known as enzyme-substrate complex The binding site is where the enzyme physically attaches itself to the substrate The active site is where the enzyme will cause a specific part of the substrate to change Cofactors/Coenzynes Some enzymes need an additional molecule to carry out the process ◦ Cofactors are inorganic ions or organic molecules that serve an enzyme helpers ◦ Coenzymes are organic molecules that function as a cofactor May be certain amino acids, nitrogenous bases, and vitamins Cofactors Turnover Number The number of molecules of substrate with which a single enzyme can react at a given time (ex. reactions/minute) is known as the turnover number ◦ Can be quite large compared to uncatalyzed reeactions ◦ Can depend on the environment Environment Temperature can have a huge impact on turnover rate ◦ a higher temperate will increase the rate of molecular motion, to a certain extent ◦ Too high of temperatures may cause the enzyme to change its shape, this is known as denaturing, where a protein structure is permanently changed Environment Optimum temperature is when the rate of formation of the enzyme-substrate complex is fastest Environment pH also affects the rate of enzymesubstrate complexes ◦ Most enzymes have an optimum pH of around 7 (neutral) However, some prefer acidic or basic conditions Competition Enzymatic competition is where there are several kinds of enzymes available to combine with the same kind of substrate molecule ◦ The substrate acetyl can be acted upon by three different enzymes: citrate synthetase, fatty acid synthetase, and malate synthetase Fig. 5.7, pg.103 Gene Regulator Proteins Gene Regulator Proteins are chemical messengers that inform the genes of the cell’s need for enzymes ◦ Gene-repressor proteins decrease protein production ◦ Gene-activator proteins will increase protein production Fig. 5.7, pg.103 Inhibitor Inhibitor is a molecule that attaches itself to an enzyme and interferes with the enzymes ability to form an enzymesubstrate complex ◦ Competitive Inhibition ◦ Negative-Feedback Inhibition Competitive Inhibition In competitive inhibition an inhibitor has a shape that is closely resembling the normal substrate of an enzyme ◦ Enzyme becomes ineffective Negative-Feedback Inhibition In negative-feedback inhibition is a process where the output of a system acts to oppose changes to the input of the system Allosteric Regulation is the regulation of an enzyme or other protein by binding an effector molecule at the protein's allosteric site (a site other than the active site) http://highered.mcgrawhill.com/olc/dl/120070/bio10.swf