Chapter 43
Mycoplasma and Ureaplasma
(黴漿菌, 尿漿菌)
Chapter 46
Chlamydiaceae (披衣菌/衣源體)
Chapter 44
Rickettsia and Orientia
Chapter 45
Ehrlichia, Anaplasma, Coxiella
Yu Chun-Keung DVM, PhD
Department of Microbiology and Immunology
Chapter 43
Mycoplasma and Ureaplasma
200 species; 16 colonize humans
Mycoplasma (黴漿菌)
M. pneumoniae
M. hominis
M. genitalium
Ureaplasma (尿漿菌)
U. urealyticum
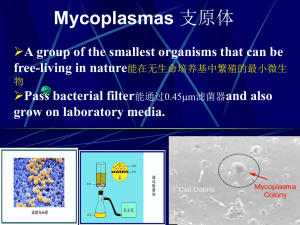
Smallest (0.1-0.3 m) and simplest freeliving bacteria (about twice the genome size
of certain large viruses)
Small, fried-egg-like colonies (except M.
pneumoniae)
Lack a cell wall
Highly pleomorphic shapes
Resistant to penicillin, cephalosporins,
vancomycin, but sensitive to tetracycline,
erythromycin.
Cell membrane contains sterols - rigid
Anaerobic (except M. pneumoniae)
Grow slowly in cell-free media, need sterols,
use glucose as a source of energy
(ureaplasmas require urea)
Epidemiology
M. pneumoniae
Strict human pathogen
Worldwide disease with no seasonal incidence
Most common in school-age children and
young adults (5-15y), but all age groups are
susceptible
Spread by respiratory droplets during coughing
episodes in close contact among classmate or
family members
U. urealyticum, M. hominis, and
M. genitalium
Infants (females) are colonized with the
agents
Carriage does not persist. Only a small
proportion of prepubertal children remains
colonized
The incidence of genital mycoplasmas
is associated with sexual activity
Sexually active men and women 15% with M.
hominis and 45-75% with Ureaplasma
Pathogenesis - M. pneumoniae
Extracellular pathogen;
infect and colonize
mucous membrane (nose,
throat, trachea, LRT).
Adheres to sialated
glycoprotein receptor (1) at the
base of cilia, (2) on surface of
RBC by means of P1 antigen.
The mechanism of cellular damage is
unknown (produce peroxide and
hemolyze RBC?)
Causes ciliostasis, destroy cilia and
ciliated epithelial cells; breakdown
clearance activity, lead to LRT infection
and persistent cough.
M. pneumoniae contains superantigen,
can attract inflammatory cells and induce
cytokine secretion (TNF, IL-1, IL-6).
Clinical disease - M. pneumoniae
Mostly asymptomatic carriage
Cause mild URT disease (acute pharyngitis),
low-grade fever, malaise, headache, dry and
nonproductive cough, persist for > 2 weeks
Tracheobronchitis with lymphocyte and
plasma cell infiltration, atypical (walking)
pneumonia
Secondary complication: hemolytic anemia,
arthritis, myocarditis, pericarditis, neurologic
abnormalities (e.g., meningoencephalitis)
Typical pneumonia - bacterial pneumonia
Abrupt, rigorous onset
Productive cough,
purulent sputum
High fever, chest pain,
stiffness in the neck
Chest consolidation
and rales.
Murray, et.al: Textbook of
Respiratory Medicine
Atypical (walking) pneumonia
Chronic in both onset
and recovery
Flulike symptomes generalized aches,
discomfort, headache,
chill, dry cough, lowgrade fever
Chest radiographs:
patchy bronchopneumonia, interstitial
pattern, not
pneumonia
Murray, et.al: Textbook of Respiratory
Medicine
Diseases caused by U. urealyticum and M.
genitalium and M. hominis
M. genitalium : nongonococcal urethritis
(NGU), pelvic inflammatory disease
U. urealyticum : NGU, pyelonephritis,
abortion, premature birth
M. hominis : pyelonephritis, postpartum
fever, systemic infection in
immunocompromised patients
Lab diagnosis
Culture of mycoplasmas is not routinely
attempted, and relatively insensitive
M. pneumoniae can grow in special medium
with animal serum (sterols), yeast extract
(nucleic acid), glucose, pH indicator, and
penicillin. Colonies have a “mulberry-shaped”.
M. hominis requires arginine for growth.
Colonies have a fried-egg appearance.
Ureaplasma requires urea for growth
Microscope: no cell wall, stain poorly, no
value
Serology – for M. pneumoniae only
Complement fixation test : high falsepositive rate
ELISA for detection of IgM and IgG Abs,
more sensitive; need dual serum samples
Cold agglutinins:
IgM Abs that bind the I antigen on human RBC
(type O) at 4°C, develop in 65% of the
patients – insensitive and nonspecific.
Treatment / Prevention / Control
M. pneumoniae: erythromycin,
tetracycline (also good for chlamydia)
Ureaplasma: use erythromycin,
resistant to tetracycline
M. hominis: resistant to erythromycin
and tetracycline, use clindamycin
Avoidance or safe sex for genital
mycoplasma
No vaccine available
Chapter 46
Chlamydia and Chlamydophila
Family Chlamydiaceae
Genus Chlamydia:
C. trachomatis (砂眼披衣菌)
Genus Chlamydophilia:
C. pneumoniae (肺炎披衣菌)
C. psittaci (鸚鵡熱披衣菌)
Chlamydiaceae
Obligate intracellular organisms
Were once considered virus, true bacteria
Contain DNA and RNA
Possess ribosomes, synthesize proteins, nucleic acid,
and lipids, but cannot synthesize ATP.
Binary fission
Susceptible to numerous antibiotics, but not to penicillin
(lack peptidoglycan)
Cell wall:
Major outer membrane protein (MOMP) – serological
variants (serovars)
Outer membrane protein 2 (OMP2) – cysteine-rich
protein, structure stability of elementary body (EB)
Unique development cycle
Two morphological distinct
forms in cytoplasmic
phagosome:
(1) elementary body (300-400
nm), resistant to harsh
environmental factors; bind to
receptors of host cells and
stimulate uptake; cannot
replicate but infectious,
(2) reticulate body (800-1000
nm), reproductive form,
metabolically active,
noninfectious.
Histologic stains can detect phagosome
with accumulated RBs (inclusion)
1. Chlamydia trachomatis (砂眼披衣菌)
Infections only occur in humans
Two biovars and 18 serovars (antigenic differences in
MOMP)
Biovars
Serovars
Trachoma A to C
D to K
LGV
L1 to L3
Disease
Trachoma
Urethritis, cervicitis,
Inclusion conjunctivitis,
Neonatal conjunctivitis,
Infant pneumonia
Lymphogranuloma
venereum (LGV)
Pathogenesis
EBs enter the body via minute abrasions and
lacerations
Trachoma serovars primarily infect nonciliated
epithelial cells (urethra, endocervix, endometrium,
fallopian tube, anorectum, respiratory tract,
conjunctiva)
LGV serovars replicate in mononuclear
phagocytes (more invasive); formation of
granuloma in lymph nodes draining the site of
primary infection, abscesses, or sinus tracts
formation
Pathogenesis
Direct destruction of cells during replication
Proinflammatory cytokine response
stimulates a severe inflammation
(accumulations of neutrophils, lymphocytes
and plasma cells).
No long-lasting immunity after infection
Re-infection induces a vigorous
inflammatory response with subsequent
tissue damage (blindness and sterility).
Trachoma (砂眼)
A chronic keratoconjuctivitis caused by serovars A,
B, Ba, C.
Diffuse follicular conjunctivitis → eyelid inward →
eyelashes abrade cornea → corneal ulceration →
pannus formation (invasion of vessels into the
cornea) → blindness
Endemic in the Middle East, North Africa, and
southern Asia (crowded and poor sanitation
regions); predominantly in children. Leading global
causes of preventable blindness (>150 million
infected, 6 million blinded).
Transmission: eye-to-eye by droplet, hands,
contaminated clothing, flies.
Urogenital infections
Venereal infections caused by serovars of D to K.
The most common sexually transmitted bacterial
disease in U.S. 2.8 million new cases annually (50
million worldwide).
In women: 80% asymptomatic as reservoir;
bartholinitis, cervicitis, endometritis, salpingitis,
urethritis, which can lead to sterility and ectopic
pregnancy.
In men: 25% asymptomatic; nongonococcal
urethritis (NGU; urethritis caused by pathogens
other than gonococcus )
Nongonococcal Urethritis (NGU)
C. trachomatis (35-50% of cases)
Ureaplasma urealyticum (10-30% of cases)
Mycoplasma hominis
Gardnerella vaginalis
Trichomonas vaginalis
Candida albicans
Dual infections of C. trachomatis and Neisseria
gonorrhoeae are common.
Post-gonococcus urethritis
Symptoms of chlamydial infection develop after
successful treatment of gonorrhea.
Reason: longer incubation period + β-lactam antibiotics
are ineffective for C. trachomatis
Reiter’s syndrome
Urethritis, conjunctivitis, polyarthritis, mucocutaneous
lesion
Usually occurs in young white man
Initiated by genital infection with C. trachomatis.
Adult Inclusion Conjunctivitis
Acute follicular conjunctivitis with
mucopurulent discharge
Mostly occur in sexually active adults
(18-30 yr) with genital infection with
serotypes A, B, Ba, D to K.
Acquired by auto-inoculation, oralgenital contact
Newborn Inclusion Conjunctivitis
25% infants acquired from mothers
with active genital infections
Swollen and hyperemic eyelids
Long (>12 months) disease course
if untreated and are at risk for
C. trachomatis pneumonia
Infant Pneumonia
A diffuse interstitial pneumonia
Occur in 10-20% infants that
exposed to the pathogen at
birth
Rhinitis → staccato cough
Lymphogranuloma venereum (LGV)
花柳性淋巴肉芽腫
A chronic sexually transmitted disease caused by
C. trachomatis L1, L2, L2a, L2b, L3.
More common in men, with male homosexuals being the
major reservoir.
Small, painless lesions (papule or ulcer) at site of infection
(genitalia). Fever, headache, myalgia.
Inflammation and swelling of regional lymph nodes
(inguinal nodes), painful buboes (橫瘻), rupture, fistulas
formation.
Proctitis is common in women.
Resolve spontaneously or progress to ulceration or genital
elephantiasis (象皮病).
Lab diagnosis
Symptomatic infections are easier to diagnosis
than asymptomatic infections as more chlamydiae
present in specimen.
Cytology – Giemsa-stained cell scrapings
Quality of the specimen is important. Specimens must
be obtained from the involved site; pus or exudate is
inadequate.
Insensitive, nonspecific
Culture – HeLa, MaCoy, Hep-2 cells
Iodine stain to detect inclusions (=RBs)
The most specific methods for diagnosis.
Sensitivity depends on quality and quantity of specimen.
Iodine-stained Chlamydia trachomatis inclusion
bodies (arrows)
Lab diagnosis
Nucleic acid amplification tests (NAATs)
Test of choice for lab diagnosis of C. trachomatis
infection
First-void urine / urethral discharge
Amplification of a specific sequence, then detecting with
a species-specific probe
Serologic tests
Limited value for adult urogenital infections, cannot
differentiate between current and past infections; good
for LGV.
CF test or EIAs: genus-specific LPS as antigen, fourfold
increase or >1:256
MIF test: species- and serovar-specific antigen (MOMPs)
T/P/C
Doxycycline for LGV
Azithromycin or doxycycline for ocular and
genital infections in adult
Erythromycin for newborn conjunctivitis
and pneumonia
Improve sanitary conditions – essential for
prevention
Safe sex practices
2. Chlamydophilia pneumoniae
Was first isolated from the conjunctiva of a
child in Taiwan - TWAR strain.
An important cause of sinusitis, pharyngitis,
bronchitis, and pneumonia.
Infection is common, especially in adults
and transmitted person-to-person by
respiratory secretions.
Clinical disease
Most infections are asymptomatic or mild persistent cough.
Cannot be differentiated with other
atypical pneumonia - Mycoplasma
pneumoniae, Legionella pneumophila, and
respiratory viruses.
Detected in atherosclerotic lesions in
blood vessels. However, the role in the
development of atherosclerosis is not
clear.
Lab diagnosis
Diagnosis is difficult
Do not grow in cell lines used for isolation of C.
trachomatis
NAATs are OK with large interlaboratary
variation.
Micro-immunofluorescence (MIF) test
The only acceptable serodiagnotic test (specific)
A single IgM titer > 1:16 or a fourfold increase in
IgG titer
T/P/C
Ubiquitous present, control is difficult
Macrolides (erythromycin), doxycycline
3. Chlamydophilia psittaci
(鸚鵡熱披衣菌)
Caused Psittacosis (parrot fever). The
natural reservoir is any species of birds
(Ornithosis,飼鳥病)
Can infect sheep, goat, cows, and humans
(zoonosis)
High risk groups: veterinarians,
zookeepers, pet shop workers, employees
of poultry industry.
Pathogenesis
Inhalation of dried bird excrement, urine, or
respiratory secretions; person-to-person
transmission is rare.
Bacteria first spread to and multiply in
reticuloendothelial cells of liver and spleen
necrosis
Then hematogenous spread to lung and other
organs via circulation
Lmphocytic inflammation in lung, edema, necrosis,
mucous plugs in bronchioles cyanosis and
anoxia
Clinical disease
Asymptomatic infection
Flu-like illness: high fever, headache,
chills, myalgia
Serious pneumonia: non-productive
cough, rales, consolidation,
CNS involvement: common (headache,
encephalitis, convulsion, coma)
GI symptoms: nausea, vomiting, diarrhea
(carditis, hepatomegaly, splenomegaly)
Diagnosis and treatment for C. psittaci
Complement fixation test of paired acute and
convalescent phase sera
Confirmed by species-specific MIF test
Treatment: tetracyclines or macrolides
No need of isolation of patients and
prophylaxia
No vaccine available
Treat birds with chlortetracycline HCl for 45
days.
Chapter 44
Rickettsia and Orientia
Chapter 45
Ehrlichia, Anaplasma, Coxiella
Rickettsia Howard Ricketts
Ehrlichia
Paul Ehrlich
Coxiella
Harold Cox
(Historically classified in Rickettsiaceae)
Order Rickettsiales
Family Rickettsiaceae
Genena Rickettsia
Orientia
Family Anaplasmataceae
Genena Ehrlichia
Anaplasma
Neorickettsia
Wolbachia
Chapter 44 Rickettsia and Orientia
Obligate intracellular parasites.
G(-) bacilli, with a minimal peptidoglycan layer
(stain poorly with Gram stain) and LPS (weak
endotoxin activity)
Maintain in animal and arthropod reservoirs (by
transovarian transmission).
Transmitted to humans by arthropod vectors
(ticks, mites, lice, fleas).
Humans are accidental hosts: acquired by
arthropod bite or contact of arthropod excreta with
abraded skin
Rickettsia (also Ehrlichia) is unstable and die
quickly outside host cells.
Coxiella highly resistant to desiccation, remain
viable in environment for months to years.
After phagocytosis
Rickettsia and Orientia: degrade phagosome
membrane by producing phospholipase, multiply in
cytoplasm and nucleus of endothelial cells
Ehrlichia and Anaplasma: multiply in cytoplasmic
vacuoles (=phagosomes) of hematopoietic cells
Coxiella: multiply in phagolysosome of monocytes
and macrophages
Important Rickettsial Diseases
Spotted fever group (17 species related to human
diseases)
R. rickettsii
R. akari
RMSF (>90%)
Rickettsialpox (100%)
Typhus group
R. prowazekii
R. typhi
O. tsutsugamushi
Epidemic typhus (40-80%)
Murine typhus (50%)
Scrub typhus (<50%)
(Parentheses: % of rash, 紅斑)
The distribution of rickettsial diseases (restricted
area or worldwide) is determined by the distribution
of the arthropod hosts/vectors.
Pathogenesis
No toxins, no immunopathology
OmpA mediated binding to endothelial cells
Rickettsia replicate in endothelial cells,
cause cell damage and blood leakage,
vasculitis, skin rash, microthrombi, focal
ischemia, hemorrhage.
Hypovolemia, hypoproteinemia, reduced
perfusion, organ failure.
Rocky mountain spotted fever
Have a restricted geographic and seasonal
distribution, corresponding to tick activity.
R. rickettsii is maintained in hard ticks (wood tick
and dog tick) by transovarian transmission.
Transmitted to humans by tick bite (need >6h to
establish infection).
High fever, chills, headache, skin rash (>90%,
extremities to trunk)
Respiratory failure, encephalitis, renal failure.
Diagnosis is urgent, the prognosis depends on the
duration of illness (identify key clinical signs – rash);
fatality 10-25% if untreated
Culture: buffy coat of blood or skin biopsy; tissue
culture or embryonated eggs (danger)
Microscopy: Giemsa stain; FA for biopsy tissue
specimens (rapid and specific)
Serology: microimmunofluorescence (MIF), detect
antibodies against MOMP and LPS antigens; both
specific and sensitive
Nucleic acid-based tests: PCR + gene sequencing
of a variety of genes
The traditional Weil-Felix test: not recommended
for use
Treatment /Prevention/Control:
Appropriate therapy would result in good
prognosis (e.g., doxycycline)
No vaccine
Prevent tick bites (can survive for as
long as 4 years without feeding)
Rickettsialpox
R. akari
Infections are transmitted to humans from rodents
reservoir by bite of infected mites (transovarian
transmission)
Cosmopolitan distribution (New York City)
Clinical disease – biphasic
Papule at site of bite, ulceration, eschar (焦痂) formation
(differentiate with cutaneous anthrax)
High fever, severe headache, chills, sweats, myalgias,
photophobia, generalized rash (100%), complete healing
2-3 wks.
Epidemic (louse-borne) typhus
流行性(蝨型)斑疹傷寒
R. prowazekii
Humans are the primary reservoir with person-toperson transmission by human louse (the
bacteria kill the lice 2 to 3 wk after infection; no
transovarian transmission).
Epidemics occur among people living in crowded,
unsanitary condition - war, famine, or natural
disaster.
High fever, severe headache, myalgias, skin rash
(20-80%), complete recovery >3 months
Brill-Zinsser Disease
Bacteria may remain for years.
A recrudescent, mild form of
epidemic typhus arising years
after the initial attack.
Diagnosis:
MIF test
T/P/C:
Tetracyclines, Chloramphenicol
Louse-control
A formaldehyde-inactivated
vaccine is available
Endemic (murine) typhus
地方性(鼠類)斑疹傷寒
R. typhi transmits to man from rodent
reservoir hosts by the bite of rat flea and
cat flea.
Endemic all over the world, primarily in
warm, humid areas.
Fever, severe headache, myalgias, chills,
skin rash (50%) on chest and abdomen
for 3 weeks.
Diagnosis:
IFA test
T/P/C:
Tetracyclines
Pest control
No vaccine
Scrub typhus 叢林斑疹傷寒
Caused by Orientia tsutsugamushi (恙蟲病立克
次體菌)
Transmitted to humans by red mites (chiggers)
Organisms are maintained in mites by
transovarian transmission.
Endemic in eastern Asia, Australia, and Japan.
Fever, severe headache, myalgias, skin rash
(<50%), spread centrifugally to extremities.
Generalized lymphadenopathy, splenomegaly,
CNS complication, heart failure
T/P/C:
Prompt treatment with
doxycycline
Avoid exposure to chiggers
No vaccine
Chapter 45
Ehrlichia, Anaplasma, Coxiella
Ehrlichia and Anaplasma
Intracellular bacteria that
lodge in phagosomes of
mononuclear and
granulocytic phagocytes,
but not RBC.
Grow cycle: three stages elementary body, reticulate
body, morulae in
phagosome (can be
detected by Giemsa or
Wright stains)
Ehrlichia inclusions (peripheral
blood smear, Wright-Giemsa)
Clinical disease
1. Human monocytic ehrlichiosis
E. chaffeensis : infect monocytes and
mononuclear phagocytes
Vector - Lone Star tick, no transovarian
transmission
Reservoir - white-tailed deer, domestic
dogs, foxes, coyotes, wolves
Humans are accident host
2. Canine granulocytic ehrlichiosis
E. ewingii: infect granulocytes
Vector - Lone Star tick
Reservoir -white-tailed deer, domestic dogs
Humans are accident host
3. Human anaplasmosis
Anaplasma phagocytophilum : infect
neutrophils, eosinophils, and basophils
Vector - Ixodes ticks
Reservoir - small mammals
Clinical disease
Fever, headache, malaise, myalgias,
leukopenia, thrombocytopenia,
elevated transaminases
Skin rash (10 to 40%)
50% patients require hospitalization,
2 to 3% mortality
Diagnosis
Diagnosis is urgent.
Stain poorly with Gram stain.
Giemsa stain of blood smear for moralae
monocytic ehrlichiosis: 10% (+)
granulocytic ehrlichiosis and anaplasmosis:
20-80% (+)
DNA amplification test: specific and
sensitive
Serology: cross-reactivity
T/P/C:
Prompt treatment with
doxycycline
No vaccine available
Avoid tick-infested areas
Coxiella burnetii
Biologically and genomically distinct from
Rickettsia; more closely related to
Legionella.
Obligate intracellular pathogen
Small cell variants (SCV): extremely resistant
to environmental stress, infectious form
Large cell variants (LCV): multiply in
phagolysosome in monocytes or macrophages
Epidemiology
Can infect mammals, birds, and ticks
Primary reservoirs: farm animals, cats,
dogs, rabbits
Ticks are vector for disease in animals
but not in humans
Zoonosis
Epidemiology
Extremely stable in harsh environmental
conditions; are able to survive in soil and milk for
months to years.
Infection is common in livestock, but symptomatic
disease is rare.
High concentrations of bacteria are present in
placenta of infected livestock.
Transmit to man by the respiratory route from
contaminated soil or ingestion of contaminated
unpasteurized milk, not from arthropod vector.
Ranchers, veterinarians, and food handlers are at
highest risk.
Pathogenesis
Target tissue is the lung, proliferate in
phagolysosomes of infected cells, then
disseminate to other organs
Undergo antigenic variation (cell wall LPS):
Infectious forms possess phase I antigen: LPS
with a complex carbohydrate, can block
antibody binding
phase II antigen is the product of the gene of
phase I antigen after deletion: a modified LPS,
expose surface proteins to antibody
Q fever
Most infections are mild or asymptomatic
Acute disease:
Mild, flulike, <5% requires hospitalization
Fever, pneumonia, hepatitis, diffuse
granulomas in involved organs
Chronic disease: subacute endocarditis
exclusively in patients with valvular heart
disease or immunosuppression; mortality
65% if untreated
Diagnosis
Serologic tests (IFA, ELISA, CF)
Acute Q fever: antibodies are developed
primarily against phase II antigen.
Chronic Q fever: antibodies against both
phase I and II antigens are elicited.
(phase I antigen: weak antigenic)
T/P/C:
Doxycycline for prolonged period
Vaccines are available
single dose with no booster
immunization for uninfected people
for adverse reaction will happen in
previously infected individuals.
96.5.14