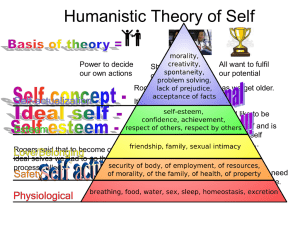
Expanded Indication - Siteman Cancer Center
advertisement
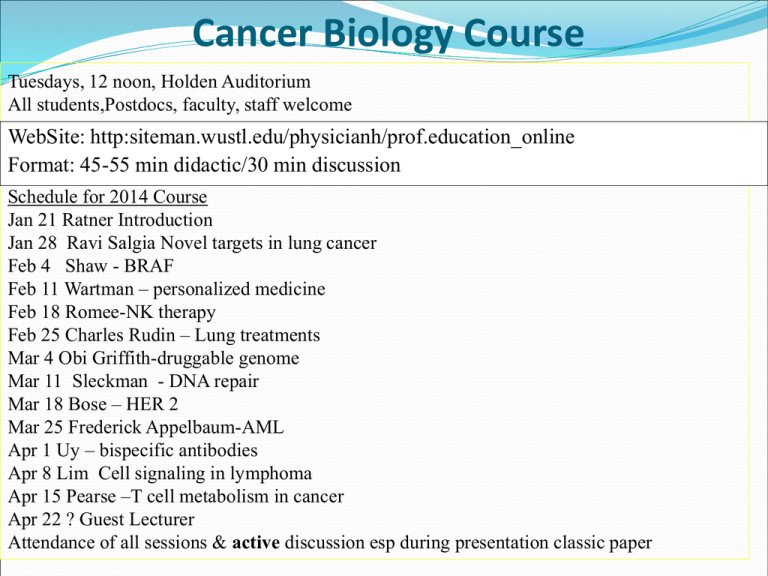
Cancer Biology Course Tuesdays, 12 noon, Holden Auditorium All students,Postdocs, faculty, staff welcome WebSite: http:siteman.wustl.edu/physicianh/prof.education_online Format: 45-55 min didactic/30 min discussion Schedule for 2014 Course Jan 21 Ratner Introduction Jan 28 Ravi Salgia Novel targets in lung cancer Feb 4 Shaw - BRAF Feb 11 Wartman – personalized medicine Feb 18 Romee-NK therapy Feb 25 Charles Rudin – Lung treatments Mar 4 Obi Griffith-druggable genome Mar 11 Sleckman - DNA repair Mar 18 Bose – HER 2 Mar 25 Frederick Appelbaum-AML Apr 1 Uy – bispecific antibodies Apr 8 Lim Cell signaling in lymphoma Apr 15 Pearse –T cell metabolism in cancer Apr 22 ? Guest Lecturer Attendance of all sessions & active discussion esp during presentation classic paper 1.6 million new cases this year 21% decrease in cancer-related deaths in men vs 1990s 12% decline in women 13 million cancer survivors alive in US Cancer-related death rates decline 1.5%/yr 580,000 Americans died of cancer in 2013 Introduction History Principles of Cancer Biology Advances in Treatment Advances in Prevention Current Social Issues See Perspectives in Nature Reviews in Cancer JCO Jan 2014, Clinical Cancer Advances deVita NEJM 2012 History of Cancer Hanahan & Weinberg Cell, 2011 Cancer – Historical Perspective 1600 BC Egyptian physician record 1st description of breast cancer 460 BC Hippocrates uses “carcinos” to describe tumors (Greek – crab) 129 AD Galen attributes cancer to black bile 1660 Mastectomy for breast cancer 1713 Ramazzini noted lack of cervical ca but increased breast ca in nuns 1775 Pott describes scrotal cancer in chimney sweeps 1838 Muller describes cancer as abnormalities of cells Boveri’s Predictions (1902) Cell-cycle checkpoints (Hemmungseinrichtung: inhibitory mechanism) that would allow cell division only when a specific external stimulus is experienced by the cell. The clonal origin of tumours. . Tumour-suppressor genes (Teilungshemmende Chromosomen), the effects of which can be overcome by external signals, and which are physically lost in progressively growing tumours. Oncogenes (Teilungsfoerdernde Chromosomen) that become amplified (im permanenten Übergewicht) during tumour development. Tumour progression from benign to malignant, involving sequential changes of increased growth-stimulatory chromosomes & loss of growth-inhibitory chromosomes. Cancer predisposition through inheritance of chromosomes (genes) that are less able to suppress malignancy. Cancer predisposition through inheritance of genes that cause aberrant mitoses. The role of wounding and inflammation in tumour promotion. Loss of cell adhesion in metastasis. Sensitivity of malignant cells to radiation therapy. Therapeutic Targeting of the Hallmarks of Cancer Pearse Sleckman AML (NEJM, 2013) • 200 adult cases • fewer mutations (13) than most other adult cancers • average of 5 are in genes that are recurrently mutated • 23 genes were significantly mutated • at least one driver in nearly all AMLs • mutation in one of 9 categories of genes relevant for pathogenesis, including transcription-factor fusions (18% of cases), the gene encoding nucleophosmin (NPM1) (27%), tumor-suppressor genes (16%), DNA-methylation-related genes (44%), signaling genes (59%), chromatin-modifying genes (30%), myeloid transcription-factor genes (22%), cohesincomplex genes (13%), and spliceosome-complex genes (14%) • Patterns of cooperation and mutual exclusivity suggested strong biologic relationships among several of the genes and categories Wartman, Griffith, Applebaum Targeting BTK with Ibrutinib in Relapsed CLL & Mantle Cell Lymphoma N Engl J Med 2013; 369:32 & 507, For CLL, at 26 mos, PFS 75%, OS 83%, For MCL PFS 13.9 mos Lim Ponatinib in Refractory CML N Engl J Med 2012; 367:2075-2088 Patients who had chronic-phase CML with the T315I mutation, 100% had a CR Blinatumomab in patients with MRD in B-lineage ALL Blood 2012 120:5185-5187 bispecific single-chain (BiTE) Abs, engages T cells for redirected lysis of CD19+ target cells ALL patients with persistent or relapsed MRD, 80% MRD RR Uy Drug Approvals 2012-3, Heme Malignancies Newly Approved Carfilzomib Pomalidomide Omacetaxine mepesuccinate Ponatinib Expanded Indications Vincristine liposome Lenalidomide Progressive myeloma For relapsed/refractory myeloma Accelerated phase CML CML or PH-pos ALL resistant to prior TKI Relapsed ALL Mantle cell lymphoma Romee Drug Approvals 2012-3, Breast Cancer Newly Approved Pertuzumab For HER2+ with trastuzumab + docetaxel TrastuzumabFor HER2+ metastatic breast cancer DM1 Expanded Indication Everolimus ER+ breast cancer with exemestane Bose CNS Tumors – mIDH1 Inhibitor for Glioma, Science, 2013 • Impairs xenograft growth • Promotes astroglial differentiation • Inhibits histone methylation CNS Tumors 6 GBM subtypes Cancer Cell, 2012 The Lancet, Volume 381, Pages 303 - 312, 26 January 2013 Regorafenib, Multifunctional inhibitor against VEGFR-2 & TIE2 OS 6·4 months in the regorafenib group vs 5·0 months in the placebo group Nature Volume: 486, Pages: 532–536 (28 June 2012) KRASm detectable in blood as early as 10 months before disease progression. Early initiation of a MEK inhibitor as a strategy for delaying or reversing drug resistance Nature Volume: 483, Pages: 100–103 (01 March 2012) BRAF(V600E) inhibition causes feedback activation of EGFR Melanoma cells express low levels of EGFR and not subject to feedback activation. Might benefit from combination therapy consisting of BRAF and EGFR inhibitors * TCGA-WNT/bCAT, RAS, PI3K, 2nd line BEV, CET adj not beneficial, ziv-aflibercept Shaw Drug Approvals 2012-3, Colorectal Cancer Newly Approved Ziv-aflibercept Resistant colorectal cancer with FOLFIRI Regorafenib Progressive colorectal cancer Expanded Indication Cetuximab 1st line KRASwt colorectal cancer Enzalutamide (MDV3100): N Engl J Med 2012; 367:1187 AR antagonist, 5x binding affinity vs bicalutamide, blocks nuclear translocation & coactivator binding, OS 18.4 vs 13.6 months in placebo group Abiraterone (17-OHase, C17:20-lyase) N Engl J Med 2013; 368:138 PFS 16.5 months with abiraterone–prednisone and 8.3 months with prednisone Radium 223 (α emitter) N Engl J Med 2013; 369:213 OS 14.0 mos vs 11.2 mos with placebo 2 protons/2 neutrons, T1/2 = 11d, bone-seeking Cabozantinib, JCO 2013; 31:412 72% regression in soft tissue lesions, 68% improvement on bone scan, 12% CR PFS 24 weeks vs 6 weeks with placebo 57% had ≥ 50% reduced alk phos and C-terminal telopeptide of collagen I 67% improved bone pain, 56% decreased narcotic use 16% grade 3 fatigue , 12% HBP, 8% hand-foot syndrome. Nature Genetics 2013;45:747 XAF1, XIAP and SRD5A1 as a predictive and actionable signature for CRPC. XAF1 = XIAP-associated factor SRD5A1 = 3-oxo-5-alpha-steroid 4-dehydrogenase 1 Cell 2013; 155:1309 Induction of glucocorticoid receptor expression as a common feature of drug-resistant tumors Drug Approvals 2012-3, GU Cancers Newly Approved Enzalutamide Docetaxel refractory prostate cancer Ra223 For bone metastases of prostate cancer Axitinib Renal cell carcinoma Expanded Indication Abiraterone Castration-resistant prostate cancer Cabozantinib delays MTC progression Sorafenib reverses RAI resistance of DTC Panitumumab improves survival of HPV-neg squamous cell cancers Freq abnormalities in squamous cell carcinomas in FGFR1, 2, DDR2, EPHA2, PI3K pathways Adeno-p53 plus chemotherapy increases survival in late-stage oral cancer 3 yr OS 88% vs 60% Salgia, Rudin Adenocarcinoma Cell. 2012 Sep 14;150:1121-34 EFGRm and KRASm in founder clones 14 fusions, including RET, ROS1 and ALK & novel metabolic enzymes Cell-cycle and JAK-STAT pathways altered with perturbations in 54 genes that may be targetable with available drugs Squamous Cell Nature 489: 519–525 (27 September 2012) Alterations in targetable oncogenic pathways: FGFR, DDR2, PI3K Melanoma - Anti–PD-1 Antibody N Engl J Med 2012; 366:2443-2454 RR 28% Melanoma ,27% Renal, 18% NSCLC Additive to CTLA4 blockade PTCHm, Vismodegib – SMOi, fatigue Weight loss, dysgeusia, m spasms ANG, CYCD1, Antiapoptosis Drug Approvals 2012-3, Skin Cancers Newly Approved Vismodegib Advanced basal cell ca Dabrafenib, BRAFm metastatic melanoma Trametinib Pazopanib for refractory soft tissue sarcoma Lancet 379:1879-1886, May 2012 OS 12·5 mos with pazopanib vs 10·7 months multitargeted tyrosine kinase inhibitor, with activity against VEGFR1,2,3 & PDGFR PD0332991 in Patients With Advanced CDK4-Amplified WellDifferentiated or Dedifferentiated Liposarcoma JCO 2013 31:2024-2028 CDK4 is amplified in > 90% of welldifferentiated (WDLS) and dedifferentiated liposarcomas (DDLS); PFS 18 weeks, 1 PR Regorafenib for refractory GIST Lancet 381:295-302, 2013 PFS was 4·8 months for regorafenib and 0·9 months for placebo Circulating DNA instead of bx Drug Approvals 2012-3, Sarcomas Expanded Indications Imatinib Regorafenib Pazopanib Denosumab Adjuvant GIST Treatment-resistant GIST Advanced soft tissue sarcoma For giant cell bone tumor Low-Dose CT Screening for Lung Cancer (NEJM 2013) A total of 53,439 eligible participants were randomly assigned to a study group (26,715 to lowdose CT and 26,724 to chest radiography) Lung cancer was diagnosed in 292 participants (1.1%) in the low-dose CT group versus 190 (0.7%) in the radiography group (stage 1 in 158 vs. 70 participants) Sensitivity and specificity were 93.8% and 73.4% for low-dose CT and 73.5% and 91.3% for chest radiography, respectively. • NIH cut existing grants by 10% • Eliminated 700 viable projects that would otherwise have been considered • NIH budget declined 22% over last decade • Number of new grants at lowest level since 1998 • If sequester not reversed, budget cuts will continue until 2021 • LOBBY CONGRESS Drug Shortages Notify FDA re drug withdrawals Economic incentives Emphasis on Quality and Value in Cancer Care Therapy only for pts who will benefit Avoid imaging/biomarkers in early stage prostate & breast ca Avoid CSFs if <20% likelihood of febrile neutropenia Continued progress depends on access to clinical trials and quality care Faster and smarter clinical trials utilizing information technology Revitalize federal funding of clinical trials Examine potential impact of health care reform on cancer disparities Assist FDA in addressing cancer drug shortages Improve advanced cancer care planning Highlight potential solutions for oncology workforce shortages Assist UN in addressing cancer crisis in developing counties