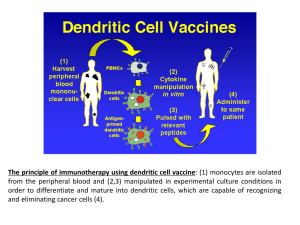
introduction to dendritic cell therapy
advertisement

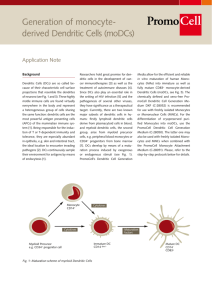
Introducing Apceden™ Topics • IMMUNITY AND CANCER • DENDRITIC CELL BIOLOGY – Development of Dendritic Cells – Why Dendritic Cells – Mechanism of Action • CLINICAL TRIALS • APCEDEN™ – Preparation – Associated Logistics IMMUNITY AND CANCER IMMUNITY AND CANCER • Clinical trials with vaccination of ex-vivo generated dendritic cells (DCs) pulsed with tumor antigens have provided a proof-ofprinciple that therapeutic immunity can be elicited in cancers • However Clinical benefit has been observed in only a fraction of cases when measured by regression of tumors in stage IV cancer • The next generation of DC vaccines are expected to generate large numbers of high avidity effector CD8+ T cells to overcome regulatory T cells and the suppressive environment established by tumors • Therapeutic vaccination protocols with improved DC vaccines in combination with chemotherapy is expected to exploit immunogenic chemotherapy regimens IMMUNOTHERAPY DC can activate Almost all Immune cells DENDRITIC CELL BIOLOGY Dendritic Cell Therapy in Cancer DEVELOPMENT OF DENDRITIC CELLS Our cells of Interest WHY DENDRITIC CELLS? There ability to migrate through tissue and act on tumors There capacity to activate naïve T cells Antigen Presenting cell MoA OF DENDRITIC CELLS ASSOCIATED TUMOR KILLING Tumor Antigen Expanding TCells Tumor Killing CLINICAL TRIALS WITH DENDRITIC CELL THERAPY Companies conducting Clinical Trials with Dendritic Cell Therapy Name of the Company Country Immunocellular Therapeutics Prima Biomed California (USA) Sydney (Australia Geron and Merix US Aastrom Bioscience Michigan (USA) Northwest USA DCPrime Amsterdam (Netherland) Dandrit Biotech Denmark Creagene Korea Dendreon Washington (USA) FEW CLINICAL TRIALS WITH DC S No. Trials Country Type of Cancer Phase No. of Patients 1 BAYLOR RESEARCH INSTITUTE USA Melanoma, neoplasm I & II Metastasis 30 2 SAMSUNG MEDICAL CENTER KOREA Prostatic cancer I & II 12 3 NATIONAL CANCER INSTITUTE USA Melanoma I 20 4 HOAG MEMORIAL HOSPITAL PRESBYTERIAN Metastatic Melanoma I & II 80 5 HERLEV HOSPITAL DENMARK Advanced Melanoma I & II 25 6 STANFORD UNIVERSITY USA Multiple Myloma I & II 30 INTERPRETING CLINICAL EFFICACY FOR DC CTs • • • • • • • Pre-mature dismissal of therapy is not suggested if ORR is not high for such a therapy Unrealistic to expect efficient immune responses to eliminate the total tumor burden in a patient with advanced cancer Analysis of improved survival benefits in randomized studies and long-term follow-up is suggested Molecular pathways or chemotherapeutics now considered active based not on only ORR but improved survival and/or time to disease progression A phase III study comparing DC Therapy with standard chemotherapy (DTIC) in melanoma patients showed insignificant ORR in DC arm but post-hoc analysis demonstrated improved survival and performance status in specific phenotype Clinical Trials results suggest that DC vaccination therapy needs to be tailored for preidentified cohorts of patients. Prolonged survival and good quality of life might be considered a therapeutic success SOME CONCLUSIONS FROM DC CTs • • • • • • • DCs are the critical decision-making cells in the immune response and an attractive target for therapeutic manipulation to enhance otherwise insufficient immune responses to tumor antigens Complexity of the DC system requires rational manipulation to achieve protective or therapeutic immunity Further research needed to analyze the immune responses induced in patients by distinct ex vivo generated DC subsets activated via different pathways Progresses made in the knowledge of DC biology as well as effector/regulatory T cell biology clearly open the avenues for development of considerably improved clinical protocols Possibility of including therapeutic vaccination of metastatic disease and preventive vaccination in patients with resected tumors The ultimate ex vivo-generated therapeutic DC vaccine will be heterogeneous and composed of several subsets, each of which will target a specific immune effector. These ex vivo strategies should help to identify the parameters for DC targeting in vivo, which lead to the next step APCEDEN™ WHAT IS Apceden™ • Apceden™ is an autologous (self) monocyte derived Dendritic Cell immunotherapy which nurtures the patient’s own mononuclear cells against cancer specific cells • Patients undergo apheresis for collection of blood monocytes. These cells are cultured and processed for the production of mature dendritic cells (DC) against the specific tumor cell type, which are harvested on Day 8. • Each dose of APCEDEN™ consists of dendritic cells (CD 80+, 83+, 86+,CD14-) more than 1 million in 15ml • 6 doses are given every 2 weeks for first 3 cycles and next 3 cycles are given every 3 weeks. Preparation of Apceden™ from Monocytes (1) Incubate for 4-6 Hrs Wash with PBS thrice Buffy Coat Adherent cells Incubate for 6 Days Incubate for 48 Hrs Preparation of Apceden™ from Monocytes (2) Isolation of Monocytes from peripheral blood Isolation of Tumor cells Generation of Immature Dendritic Cells Lysate Preparation Loading of Dendritic cells with whole cell Tumor Lysate Patient The APCEDENTM is to be administered to the patient intravenously Mature antigen presenting Dendritic Cells Associated Logistics NutriprepTM Registration 8 Days APCEDEN™ Dendritic Cells Salubrious Autologous Target Specific Safe Minimal Toxicity