Complimentary Slide Presentation.
advertisement

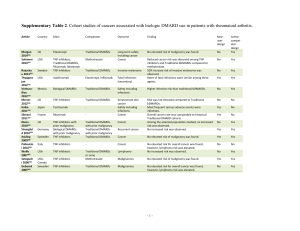
Rheumatoid Arthritis in Practice An Expert Commentary With Roy Fleischmann, MD A Clinical Context Report Rheumatoid Arthritis in Practice Jointly Sponsored by: and Rheumatoid Arthritis in Practice Expert Commentary Supported in part by educational grants from Abbott and Centocor Ortho Biotech. Rheumatoid Arthritis in Practice Clinical Context Series The goal of this series is to provide up-todate information and multiple perspectives on the pathogenesis, symptoms, risk factors, and complications of rheumatoid arthritis as well as current and emerging treatments and best practices in the management of rheumatoid arthritis. Rheumatoid Arthritis in Practice Clinical Context Series Target Audience Rheumatologists, pain management specialists, geriatricians, family practice/primary care physicians, nurses, nurse practitioners, physician assistants, pharmacists. and other healthcare professionals involved in the management of patients with rheumatoid arthritis. Activity Learning Objective CME Information: Physicians Statement of Accreditation This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of the University of Pennsylvania School of Medicine and MedPage Today. The University of Pennsylvania School of Medicine is accredited by the ACCME to provide continuing medical education for physicians. CME Information Credit Designation The University of Pennsylvania School of Medicine Office of CME designates this enduring material for a maximum of 0.5 AMA PRA Category 1 Credits.™ Physicians should claim only the credit commensurate with the extent of their participation in the activity. CME Information: Physicians Credit for Family Physicians MedPage Today "News-Based CME" has been reviewed and is acceptable for up to 2098 Elective credits by the American Academy of Family Physicians. AAFP accreditation begins January 1, 2011. Term of approval is for one year from this date. Each article is approved for 0.5 Elective credits. Credit may be claimed for one year from the date of each article. CE Information: Nurses Statement of Accreditation – Projects In Knowledge, Inc. (PIK) is accredited as a provider of continuing nursing education by the American Nurses Credentialing Center’s Commission on Accreditation. – Projects In Knowledge is also an approved provider by the California Board of Registered Nursing, Provider Number CEP-15227. – This activity is approved for 0.50 nursing contact hours. DISCLAIMER: Accreditation refers to educational content only and does not imply ANCC, CBRN, or PIK endorsement of any commercial product or service. CE Information: Pharmacists Projects In Knowledge® is accredited by the Accreditation Council for Pharmacy Education (ACPE) as a provider of continuing pharmacy education. This program has been planned and implemented in accordance with the ACPE Criteria for Quality and Interpretive Guidelines. This activity is worth up to 0.5 contact hours (0.05 CEUs). The ACPE Universal Activity Number assigned to this knowledge-type activity is 0052-9999-11-1383-H04-P. Discussant Roy Fleischmann, MD Clinical Professor of Medicine University of Texas Southwestern Medical Center Dallas, Texas Disclosure Information Roy Fleischmann, MD, has disclosed that he has no relevant financial relationships or conflicts of interest to report. Disclosure Information Dori F. Zaleznik, MD, Associate Clinical Professor of Medicine, Harvard Medical School, Boston; Nancy Walsh; and Dorothy Caputo, MA, RN, BC-ADM, CDE, Nurse Planner; have disclosed that they have no relevant financial relationships or conflicts of interest with commercial interests related directly or indirectly to this educational activity. The staff of The University of Pennsylvania School of Medicine Office of CME, MedPage Today, and Projects In Knowledge have no relevant financial relationships or conflicts of interest with commercial interests related directly or indirectly to this educational activity. Rheumatoid Arthritis: Scope of the Problem • 1,293,000 Americans ages 18 and older have rheumatoid arthritis • Across most developed countries the incidence is similar, at approximately 0.5% to 1% of adults Reference: Helmick C, et al “Estimates of the prevalence of arthritis and other rheumatic conditions in the United States” Arthritis Rheum 2008; 58: 15-25. The Methotrexate Era • Before the mid 1980s treatment of active RA consisted primarily of gold or penicillamine • RA is frequently severe and debilitating; the side effects of DMARDs were problematic • In 1988 methotrexate was approved for use in RA which was a quantum leap forward • Methotrexate remains the cornerstone of therapy of RA today The Tumor Necrosis Factor (TNF) Era • The first biologic for RA, etanercept (Enbrel), was approved in the U.S. in 1998 • There are now five TNF inhibitors on the market • The TNF inhibitors were a significant addition to our armamentarium which has led to dramatic improvements in patient outcomes Definitions of Remission in RA • The Disease Activity Score [28 joints] (DAS28) is a disease measure which utilizes the number of tender and swollen joints in a 28 joint count, the erythrocyte sedimentation rate or the C-reactive protein (CRP), and the patient general health on a visual analog scale (0-100). DAS28 (ESR) remission is a score below 2.6 Definitions of Remission in RA (cont’d) • The Simplified Disease Activity Index (SDAI) is a disease measure which adds the tender and swollen joints (28 count), CRP, patient’s global disease activity, and physician’s global assessment. SDAI remission is a score <3.3 • The Clinical Disease Activity Index (CDAI) is similar to the SDAI except no laboratory test measurements are included. Remission is a score below 2.8 Definitions of Remission in RA (cont’d) • Recently, the American College of Rheumatology and the European League Against Rheumatism developed provisional remission criteria for use in clinical trials. These criteria include either a score of ≤3.3 on the SDAI or scores no higher than 1 on tender joint count, swollen joint count, patient global assessment, and CRP in mg/dL Reference: Felson D, et al “American College of Rheumatology/European League Against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials” Arthritis Rheum 2011; 63: 573-586. Initiating Treatment • Start with methotrexate, unless contraindicated • Combine with folic acid (at least 1 mg per day) • Consider using low-dose corticosteroids, if necessary • Reassess patient in eight weeks by measuring DAS28, CDAI, SDAI and/or RAPID3 Initiating Treatment (cont’d) • If patient responds (such as with a decrease in DAS28 of 1.2 or more) continue on methotrexate for an additional eight weeks • With further response, continue methotrexate and taper steroids Approved Biologic Agents • Etanercept (Enbrel) is a fusion protein given as a weekly, 50-mg subcutaneous injection in RA • Infliximab (Remicade) is a chimeric monoclonal antibody given in doses of 3 mg/kg to 10 mg/kg every four to eight weeks • Adalimumab (Humira) is a fully human monoclonal antibody given as a subcutaneous injection of 40 mg every other week and occasionally weekly Approved Biologic Agents (cont’d) • Certolizumab pegol (Cimzia) is a pegylated Fab’ fragment of a humanized monoclonal antibody, given in subcutaneous doses of 200 mg every other week or 400 mg a month after a loading dose • Golimumab (Simponi) is another human monoclonal antibody given subcutaneously in doses of 50 mg once a month Continuing Treatment • If response is inadequate, consider adding a TNF inhibitor • Assess response at 12 weeks; if sufficient, continue anti-TNF treatment until disease activity is low • If the response is not adequate after 12 weeks, consider switching to another TNF inhibitor or a different class of biologic Biologic Drugs for RA-2 • Several other types of biologic drugs (e.g., a T-cell costimulation modulator and monoclonal antibodies against IL-6 and CD20) also have been developed, and are typically given to patients who do not fully respond to a TNF inhibitor. As with the TNT inhibitors, they generally are given in combination with methotrexate Biologic Drugs for RA-2 (cont’d) • Abatacept (Orencia) is a selective costimulation modulator of T cells given as a monthly intravenous infusion in doses ranging from 500 to 1,000 mg depending on patient body weight, after a loading dose • Tocilizumab (Actemra) is a humanized monoclonal antibody that binds to the interleukin (IL)-6 receptor. It is given by intravenous infusion every four weeks in doses of 4 to 8 mg/kg Biologic Drugs for RA-2 (cont’d) • Rituximab (Rituxan) is a chimeric monoclonal antibody against the CD20 protein on the surface of B cells. This drug is given as two 1,000 mg infusions; the frequency of repeat therapy is not clear but certainly not before 16 weeks after the last infusion Common Adverse Effects of the Biologic Drugs • Etanercept: Infections and injection site reactions • Infliximab: Upper respiratory tract infections, sinusitis, pharyngitis, infusion reactions, headache, and abdominal pain • Adalimumab: Upper respiratory tract infections, sinusitis, injection site reactions, headache, and rash Common Adverse Effects of the Biologic Drugs (cont’d) • Certolizumab pegol: Upper respiratory tract infections, rash, and urinary tract infections • Golimumab: Upper respiratory tract infections, nasopharyngitis • Abatacept: Headache, upper respiratory tract infections, nasopharyngitis, and nausea Common Adverse Effects of the Biologic Drugs (cont’d) • Tocilizumab: Upper respiratory tract infections, nasopharyngitis, headache, hypertension, increased alanine transaminase levels • Rituximab: Upper respiratory tract infection, nasopharyngitis, urinary tract infection, bronchitis. Other potentially important events include infusion reactions, serious infections, cardiovascular events and, progressive multifocal leukoencephalopathy The Patient’s Choice • Explain the similarities and differences of the drugs • Mode of administration and timing differ among the biologics • Efficacy and side effects are generally similar • Encourage the patient to choose a treatment based on personal preference unless their insurance plan specifies which therapies are permitted Serious Infections • Most viral infections are not a concern • Bacterial infections Treat until resolved Withhold biologic therapy until resolved Reconsider biologic therapy if infections recur • Screen for tuberculosis and watch for opportunistic infections Other Safety Issues • Avoid TNF inhibitors in patients with congestive heart failure NYHA Class III/IV or demyelinating disease • Lymphomas and other malignancies have been reported in patients on biologics but don’t appear to be a major clinical concern • Patients should take precautions against skin cancer New Drugs in the Pipeline • Additional IL-6 antibodies • Other anti-CD20 antibodies • Kinase inhibitors JAK inhibitors SYK inhibitors Phase III Study of Ofatumumab Responses at week 24 • Results of a phase III trial of the monoclonal antibody ofatumumab, which targets a different CD20 epitope than rituximab, were presented as an abstract at the 2011 EULAR meeting • A total of 260 patients with active RA who had not previously been treated with biologic drugs were enrolled and randomized to receive two infusions (each 700 mg) or placebo along with methotrexate (P<0.001) Source: Taylor P, et al “Ofatumumab, a fully human anti-CD20 MAB in the treatment of biologic-naïve rheumatoid arthritis patients: a randomized, double-blind, placebocontrolled clinical trial” EULAR 2011; Abstract OP0019. Monotherapy With an Oral JAK Inhibitor • A phase 3 study of monotherapy with the oral JAK inhibitor tofacitinib randomized 610 patients with active RA to 5 or 10 mg of tofacitinib twice daily or placebo • After three months of treatment, significantly more patients on the active treatment had ACR20 responses (P<0.0001) Source: Fleischmann R, et al “Phase 3 study of oral JAK inhibitor tasocitinib (CP690,550) monotherapy in patients with active rheumatoid arthritis”; ACR 2011; Abstract L8. Summary At the end of this activity, participants should understand: An estimated 1.3 million American adults have RA Dramatic changes have occurred in the treatment and outcome of patients with the development of new therapies, beginning with the TNF inhibitors in the late 1990s The goal of treatment today is remission, which has been defined in several ways, including the DAS28 score, SDAI, CDAI, and a provisional ACR/EULAR definition Summary Most patients begin treatment with methotrexate, which results in remission at 16 weeks in about one-third of patients If patients continue to have disease activity, the usual next step is to treat with a TNF inhibitor There are currently five TNF inhibitors on the market, which vary in mode and frequency of administration. The drugs are generally similar in efficacy and side effect profiles Summary A safety concern with the biologic drugs is the potential for serious infections, so monitoring is needed Other options also are now available for patients who don’t respond to TNF inhibitors or who experience adverse events. The choice of which drug to use may reflect the decision of the patient’s insurance company or the patient’s preference A number of new drugs are in the pipeline such as JAK and SYK inhibitors, and studies now under way should help clarify their therapeutic niches