Centralized Non-Formulary Processing
advertisement
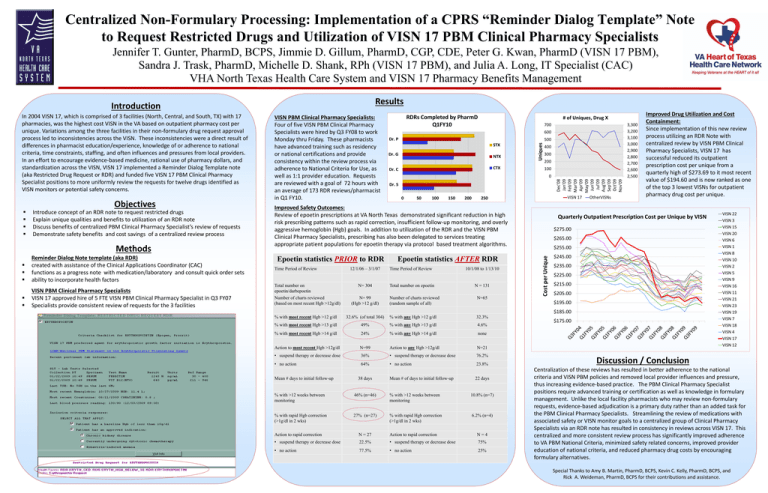
Centralized Non-Formulary Processing: Implementation of a CPRS “Reminder Dialog Template” Note to Request Restricted Drugs and Utilization of VISN 17 PBM Clinical Pharmacy Specialists Jennifer T. Gunter, PharmD, BCPS, Jimmie D. Gillum, PharmD, CGP, CDE, Peter G. Kwan, PharmD (VISN 17 PBM), Sandra J. Trask, PharmD, Michelle D. Shank, RPh (VISN 17 PBM), and Julia A. Long, IT Specialist (CAC) VHA North Texas Health Care System and VISN 17 Pharmacy Benefits Management Results Objectives Introduce concept of an RDR note to request restricted drugs Explain unique qualities and benefits to utilization of an RDR note Discuss benefits of centralized PBM Clinical Pharmacy Specialist’s review of requests Demonstrate safety benefits and cost savings of a centralized review process Methods Reminder Dialog Note template (aka RDR) created with assistance of the Clinical Applications Coordinator (CAC) functions as a progress note with medication/laboratory and consult quick order sets ability to incorporate health factors VISN PBM Clinical Pharmacy Specialists VISN 17 approved hire of 5 FTE VISN PBM Clinical Pharmacy Specialist in Q3 FY07 Specialists provide consistent review of requests for the 3 facilities RDRs Completed by PharmD Q1FY10 # of Uniques, Drug X 700 600 Dr. P 500 STX Dr. G NTX CTX Dr. C 400 300 200 100 0 Dr. S 0 50 100 150 200 VISN 17 250 Improved Safety Outcomes: Review of epoetin prescriptions at VA North Texas demonstrated significant reduction in high risk prescribing patterns such as rapid correction, insufficient follow-up monitoring, and overly aggressive hemoglobin (Hgb) goals. In addition to utilization of the RDR and the VISN PBM Clinical Pharmacy Specialists, prescribing has also been delegated to services treating appropriate patient populations for epoetin therapy via protocol based treatment algorithms. Epoetin statistics PRIOR to RDR Time Period of Review Total number on epoetin/darbepoetin Number of charts reviewed (based on most recent Hgb >12g/dl) 12/1/06 – 3/1/07 N= 304 N= 99 (Hgb >12 g/dl) Epoetin statistics AFTER RDR Time Period of Review Total number on epoetin Number of charts reviewed (random sample of all) 10/1/08 to 1/13/10 N = 131 N=65 % with most recent Hgb >12 g/dl 32.6% (of total 304) % with any Hgb >12 g/dl 32.3% % with most recent Hgb >13 g/dl 49% % with any Hgb >13 g/dl 4.6% % with most recent Hgb >14 g/dl 24% % with any Hgb >14 g/dl none Action to most recent Hgb >12g/dl N=99 Action to any Hgb >12g/dl N=21 • suspend therapy or decrease dose 36% • suspend therapy or decrease dose 76.2% • no action 64% • no action 23.8% Mean # days to initial follow-up 38 days 3,300 3,200 3,100 3,000 2,900 2,800 2,700 2,600 2,500 Dec'08 Jan'09 Feb'09 Mar'09 Apr'09 May'09 Jun'09 Jul'09 Aug'09 Sep'09 Oct'09 Nov'09 VISN PBM Clinical Pharmacy Specialists: Four of five VISN PBM Clinical Pharmacy Specialists were hired by Q3 FY08 to work Monday thru Friday. These pharmacists have advanced training such as residency or national certifications and provide consistency within the review process via adherence to National Criteria for Use, as well as 1:1 provider education. Requests are reviewed with a goal of 72 hours with an average of 173 RDR reviews/pharmacist in Q1 FY10. Mean # of days to initial follow-up 22 days % with >12 weeks between monitoring 46% (n=46) % with >12 weeks between monitoring 10.8% (n=7) % with rapid Hgb correction (>1g/dl in 2 wks) 27% (n=27) % with rapid Hgb correction (>1g/dl in 2 wks) 6.2% (n=4) Action to rapid correction N = 27 Action to rapid correction N=4 • suspend therapy or decrease dose 22.5% • suspend therapy or decrease dose 75% • no action 77.5% • no action 25% OtherVISNs Improved Drug Utilization and Cost Containment: Since implementation of this new review process utilizing an RDR Note with centralized review by VISN PBM Clinical Pharmacy Specialists, VISN 17 has successful reduced its outpatient prescription cost per unique from a quarterly high of $273.69 to it most recent value of $194.60 and is now ranked as one of the top 3 lowest VISNs for outpatient pharmacy drug cost per unique. Quarterly Outpatient Prescription Cost per Unique by VISN $275.00 $265.00 $255.00 Cost per Unique In 2004 VISN 17, which is comprised of 3 facilities (North, Central, and South, TX) with 17 pharmacies, was the highest cost VISN in the VA based on outpatient pharmacy cost per unique. Variations among the three facilities in their non-formulary drug request approval process led to inconsistencies across the VISN. These inconsistencies were a direct result of differences in pharmacist education/experience, knowledge of or adherence to national criteria, time constraints, staffing, and often influences and pressures from local providers. In an effort to encourage evidence-based medicine, rational use of pharmacy dollars, and standardization across the VISN, VISN 17 implemented a Reminder Dialog Template note (aka Restricted Drug Request or RDR) and funded five VISN 17 PBM Clinical Pharmacy Specialist positions to more uniformly review the requests for twelve drugs identified as VISN monitors or potential safety concerns. Uniques Introduction $245.00 $235.00 $225.00 $215.00 $205.00 $195.00 $185.00 $175.00 VISN 22 VISN 3 VISN 15 VISN 20 VISN 6 VISN 1 VISN 8 VISN 10 VISN 2 VISN 5 VISN 9 VISN 16 VISN 11 VISN 21 VISN 23 VISN 19 VISN 7 VISN 18 VISN 4 VISN 17 VISN 12 Discussion / Conclusion Centralization of these reviews has resulted in better adherence to the national criteria and VISN PBM policies and removed local provider influences and pressure, thus increasing evidence-based practice. The PBM Clinical Pharmacy Specialist positions require advanced training or certification as well as knowledge in formulary management. Unlike the local facility pharmacists who may review non-formulary requests, evidence-based adjudication is a primary duty rather than an added task for the PBM Clinical Pharmacy Specialists. Streamlining the review of medications with associated safety or VISN monitor goals to a centralized group of Clinical Pharmacy Specialists via an RDR note has resulted in consistency in reviews across VISN 17. This centralized and more consistent review process has significantly improved adherence to VA PBM National Criteria, minimized safety related concerns, improved provider education of national criteria, and reduced pharmacy drug costs by encouraging formulary alternatives. Special Thanks to Amy B. Martin, PharmD, BCPS, Kevin C. Kelly, PharmD, BCPS, and Rick A. Weideman, PharmD, BCPS for their contributions and assistance.







