Paracetamol plus ibuprofen for the treatment of fever in children
advertisement

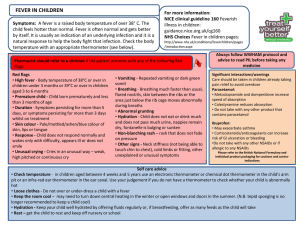
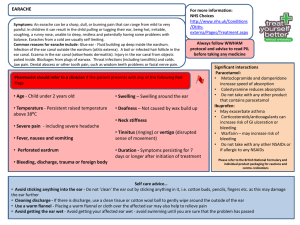
Paracetamol plus Ibuprofen for the treatment of fever in children (PITCH): RCT Journal Club 1st May 2012 SCH Aim of the study To determine whether paracetamol plus ibuprofen are superior to either drug alone for increasing time without fever and for the relief of fever associated discomfort in febrile children managed at home falcon Tue 12-Aug-08 16:45:10 • I wouldn't give both simultaneously to a child Seeline Wed 07-Mar-12 10:33:18 • I have been told by GP that you can give ibuprofen in between Calpol ie both are 4-6 hourly doses so just give the other 2-3 hours after the first and keep alternating allsquareknickersnofurcoat Mon 07-Mar-11 00:35:30 • I was told by the doctor that you could take full doses of both as they do different things? Not sure how it works for a child though. Rebelcountycailin Sat 07-Jan-12 20:49:36 • Please, please help - thinking I was holding the bottle of Paracetamol, I gave my 2.2yo a double dose of Ibuprofen by accident. Do I need to take him to A&E? He is fine and running around at the moment Gigondas Sat 07-Jan-12 21:05:06 • You can give paracetamol with nurofen. Sounds like he has had double the dose so I would take him to A and E to be sure as they can give him something to mop it up Rebelcountycailin Sat 07-Jan-12 21:03:27 • He is busy eating brioche rolls and dry cheerios and he's had some water What do you do? What advice to you give? So....let’s get to the bottom of this... What is better...? • • • • PICO Current practice Methods and Intervention Determine the validity and reliability of the paper chosen using the CASP tool • Discuss if it changes our practice PICO Patient: Children ages between 6 months and 6 years with axillary temperatures of at least 37.8o and up to 41o Intervention: Advice on physical measures to reduce temperatures and the provision of, and advice to give, paracatamol plus ibuprofen, ibuprofen or paracetamol alone. Comparison: Placebo Outcome: Time without fever in the first 4 hours after dose Current practice: This is what NICE suggest... • Consider either paracetamol or ibuprofen as an option if the child appears distressed or is unwell. • Take the views and wishes of parents and carers into account when considering the use of antipyretic agents. • Do not administer paracetamol and ibuprofen at the same time, but consider using the alternative agent if the child does not respond to the first drug. Literature Search • Medline – Paracetamol and Ibuprofen and Fever – Limited to Clinical Trial, English, 0-18 years old – 44 results • Secondary care, single time points, conflicting or insufficient evidence Paracetamol plus ibuprofen for the treatment of fever in children (PITCH): randomised controlled trial Alastair D Hay et al BMJ 2008;337:a1302 Methods • Recruited and followed up children between January 2005 and May 2007 • Recruitment from all NHS services – research nurses present in waiting rooms, contactable by fax or phone via local GPs, local promotion in media. • Once idenitifed, parents of eligible children were contacted and met with another nurse to verify eligibilty and written consent obtained. • The nurse then called automated randomised telephone service and randomised to one of three arms • Followed up at 24 hours, 48 hours and Day 5 Inclusion and Exclusion • Inclusion criteria – 6 months – 6 years – Unwell with temperature of at least 37.8o and up to 41o – As a result of illness that could be managed at home • Exclusion criteria – – – – – – Required hospital admission Clinically dehydrated Recently participated in another trial Previously participated in PITCH Known allergy, intolerance, contraindication to drugs Chronic neuro, cardiac, respiratory (expect asthma) renal or liver disease – Parents who could not read or write in English Intervention • All parents given two bottles – either both active or one active and one placebo • Given the difference in dosing schedules parents knew which one was paracetamol/placebo and ibuprofen/placebo • Dose determined by weight and either QDS or TDS for 48 hours • In the first 4 hours after the drugs were given was the ‘efficacy period’ • The drugs were given regularly from 4 to 24 hours – ‘proactive period’ • From 24 to 48 hours the parents were told to give the drugs in response to their children’s symptoms – ‘reactive period’ • The drugs were then retrieved and parents adviced to use OTC medication until day 5 Outcomes • All outcome measures were timed in relation to the first dose of antipyretics • Axillary temperature probe for the first 24 hours, measuring temperature every 30 seconds • Symptom diary, standard axillary thermometer • Primary outcomes – – Number of minutes without fever in the first four hours – Proportion of children reported as ‘normal’ on the discomfort scale at 48 hours (normal, not quite normal, some distress, very distressed) • Secondary outcomes – – Collected at three time points • In the first 24 hours the time to temperature first falling below 37.2 (fever clearance) • Time spent without fever over 24 hours and the proportion of children without fever associated symptoms • At 48 hours and day 5 the study obtained data on fever associated symptoms measured by parents CASP Are the results of the trial valid? • 1. Did the trial address a clearly focused issue? – Yes • 2. Was the assignment of patients to treatments randomized? – Yes, telephone service with allocation to one of the three arms of the trial minimised by age, severity of fever, discomfort scale, previous duration of fever and current antibiotic use • 3. Were all of the patients who entered the trial properly accounted for at its conclusion? – N=51 and n=50 in the flow chart but n=52 in the tables – Children reported to be ‘normal’ in table 1 denominators may vary owing to missing data ‘in most cases fewer than 4 children’ CASP Are the results of the trial valid? • 4. Were patients, healthcare workers and study personnel blind to treatment? – Healthcare workers and study personnel – yes – Parents – know which was ibuprofen/placebo and which was paracetamol/placebo due to the dosing regimes • 5. Were the groups similar at the start of the trial? – Mostly yes – table 1 Baseline characteristics – More boys in Ibuprofen alone group – Initial discomfort scale ‘crying or very distressed’ more in in the paracetamol plus ibuprofen group CASP Are the results of the trial valid? • 6. Aside from the experimental intervention, were the groups treated equally? – Yes, all groups received same treatment and follow up – Deviation from the protocol occurred in 13 children who received a fifth dose of paracetamol and 18 children who received a fourth dose of ibuprofen – included in the results CASP What are the results? • 7. How large was the treatment effect? • 8. How precise was the estimate of the treatment effect? Results • Summary: – Primary outcome • Time without fever in the first four hours in the paracetamol plus ibuprofen group was significantly more than paracatamol alone, and similarly with ibuprofen alone compared to paracetamol alone. • Suggesting little difference in giving paracetamol plus ibuprofen than giving ibuprofen alone • Wide confidence interval and large P values suggest no difference between all three arms of the trial for being ‘normal’ at 48 hours Results Figure 3 Results • Summary – Secondary outcome • Fever clearance (the time to temperature first falling to below 37.2) was quickest in the paracetamol plus ibuprofen than paracetamol alone, and that ibuprofen was superior to paracetamol alone • No evidence for fever associated symptoms Results Table 4 CASP Will the results help locally? • 9. Can the results be applied to the local population? – Yes, broad selection of patients accessing different types of healthcare • 10. Were all clinically important outcomes considered? – Yes, including adverse effects • 11. Are the benefits worth the harms and costs? – Separate paper suggests there is no strong evidence of a difference in cost between the treatments – Risk of unintentionally exceeding the maximum dose – 6 – 13% in just this trial! Summary • So, the bottom line is... Well designed study but no significant effect found on parent’s opinion of discomfort in their children Ibuprofen is better than paracetamol alone in the first 4 hours Consider adding in paracetamol once considering the risks to reduce fever over 24 hours Does this change your practice? Possible Limitations • Temperature of 37.8 – arbitary figure • Parents not fully blinded to treatment and ability to compare at home if they wanted to –might affect parental recording of discomfort, but not of time without fever • Dose by weight • Axillary themomter