File
advertisement

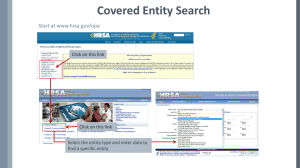
Optimizing The 340B Program Promoting Integrity, Access, & Value To deliver clinically and cost-effective pharmacy services This educational product created by: Health Resources and Services Administration | Office of Pharmacy Affairs 340B Peer-to-Peer Program 340B 101: The Basics Purpose of Activity The purpose of this module is to illustrate the history, intent and statutory principles of the 340B Drug Pricing Program. Topic Guide Intent of the program 340B pricing determination Entity eligibility Entity enrollment procedure Program requirements and prohibitions Program guidance and policy Patient eligibility determination Drug-delivery options Available resources Creation of the 340B Program Certain safety net covered entities Outpatient drugs 340B Program Price discounts Required for all manufacturers in Medicaid Intent of the 340B Program Stretch scarce federal resources1 Reach more eligible patients1 Reduce price of pharmaceuticals for patients Expand services offered to patients Provide more comprehensive services1 Provide services to more patients 1. HR Rep No. 102–384, pt 2, at 12 (1992). 340B Program Evolution 2010 2004 1996 1992 1993 Contract Pharmacy, 1st Guidelines Patient Definition 340B Statute Vendors Affordable Care Act 1st Proposed Regulations 340B Price 340B Drug Pricing Program 25%–50% of the average wholesale price The 340B price is actually considered a “ceiling” price Can offer subceiling prices Drug Manufacturers 340B Covered Drugs • Outpatient prescription drugs • Over-the-counter drugs (with prescription) • Clinic-administered drugs • Biologics (prescription) • Insulin 11 • Inpatient drugs • Vaccines 340B Eligible Entities › Hospital Types › Federal Grantees • Comprehensive hemophilia treatment centers • • • • • • • Federally qualified health centers/lookalikes • Urban/638 health center • Ryan White programs Disproportionate share hospitals Children’s hospitals* Critical access hospitals* Free-standing cancer hospitals* Rural referral centers* Sole community hospitals* • Sexually transmitted disease/tuberculosis • Title X family planning *340B eligible through Section 7101 of the Affordable Care Act (ACA) 11 Hospital Eligibility Criteria Non-profit/ Govt. Contract DSH% Group Purchasing Organization (GPO) * Prohibition Orphan Drug* Applies? Disproportionate Share Hospital (DSH) Yes >11.75% Yes No Children’s Hospital (PED) Yes >11.75% Yes No Free-standing Cancer Hospital (CAN) Yes >11.75% Yes Yes Critical Access Hospital (CAH) Yes N/A No Yes Rural Referral Center (RRC) Yes >8% No Yes Sole Community Hospital (SCH) Yes >8% No Yes Entity Type *340B eligible through Section 7101 of the Affordable Care Act (ACA) Hospital Outpatient Facilities › In order for outpatient facilities to become eligible for the 340B Program: – The outpatient facility must be an integral part of the hospital – The outpatient facility must be included as reimbursable on the covered entity’s most recently filed Medicare Cost Report – To register additional outpatient facilities, complete the online Register an Outpatient Facility registration at: http://opanet.hrsa.gov/OPA/CERegister.aspx 11 340B Enrollment Procedure http://opanet.hrsa.gov/OPA/CERegister.aspx Determine Eligibility Enroll online Submit Forms to OPA as directed Await decision from OPA 340B Implementation › Ensure entity is listed correctly in the OPA 340B database › Set up an account with wholesaler using 340B ID for purchasing • Wholesalers will not ship discounted drugs unless 340B ID is an exact match to the 340B database › Prepare operational and logistical monitoring, auditing, and compliance processes and procedures › Utilize available resources • Prime Vendor Program for sub-ceiling 340B pricing, value-added services and for technical assistance 340B Prohibitions and Requirements Prohibitions Diversion Duplicate Discounts Duplicate Discount Prohibition Duplicate Discount Accessing the 340B discount AND Medicaid Rebate on the same drug Carve In (use 340B with Medicaid) Carve Out (do not use 340B with Medicaid) • Medicaid Exclusion File at: http://opanet.hrsa.gov/opa/CEMedicaidExtract.aspx • Medicaid Exclusion Tutorial at: http://www.hrsa.gov/opa/medicaidexclusion.htm • State policies • Entities should contact their state Medicaid offices for state-specific requirements for using 340B with Medicaid patients. Fed Regist. 2000;65(51):13983–4. Diversion Prohibition › Diversion occurs when: • A drug is provided to an individual who is not a patient of that entity • Required to follow patient definition guidelines1 • A drug is dispensed in an area of a larger facility that is not eligible (e.g., an inpatient service, a noncovered clinic) • Entities should enroll all eligible outpatient or satellite sites 1. Fed Regist.1996;61(207):55156–8. GPO Prohibition › GPO prohibition prohibits certain entities from purchasing any covered outpatient drugs through a GPO or other group-purchasing arrangement, even if items are available at a lower price through the GPO. DSHs GPO Prohibition Only Applies to PEDs CANs Hospitals can continue to purchase all products for inpatient operations through a GPO, even if their outpatient departments participate in 340B. The Orphan Drug Exclusion › The orphan drug exclusion prohibits certain entities from purchasing orphan drugs at 340B discount prices. CAHs Orphan Drug Exclusion Only Applies to SCHs RRCs CANs › The Orphan Drug Product Designation Database can be found at: › http://www.accessdata.fda.gov/scripts/opdlisting/oopd/index.cfm 340B Guidance and Policy Patient Definition http://www.hrsa.gov/opa/federalregister.htm Contract Pharmacy Federal Register Notice Outpatient Facilities Audits and Dispute Resolution Duplicate Discounts 340B Proposed Regulations Civil Monetary Penalties Regulations (Proposed) Dispute Regulation Patient Definition For eligibility, three components must always be considered regarding the individual and his/her associated prescription: Entity has established a relationship and maintains records of care Patient must receive health-care services from health-care professional employed/contracted with entity, and entity must maintain responsibility for the care provided Patient receives health care consistent with range of services from the covered entity (hospitals are exempt) Fed Regist. 1996;61(207):55156–8. Drug Delivery Contract Pharmacies › 340B Program allows entities to have multiple contract pharmacies for increased patient access to cost-effective pharmaceuticals › Covered entity purchases the drug, but “ship to/bill to” procedure may be used › Covered entity retains legal title to all drugs purchased under 340B and must pay for all 340B drugs › Fed Regist. 2010;75(43):10272–9. 340B Usage Considerations Federal grantees • Scope of grant limitations Hospital facilities • Integral part of the hospital • On most recently filed cost report 11 340B Program Resources Integrity Program integrity assures stakeholders that the 340B Program’s intent is being met and that rules are being followed. Access Access to services under the 340B Program is important because it ensures that entities and their patients have the means to fully utilize the program’s benefits. Value The value that program participation brings to entities is essential for stretching scarce entity resources. Office of Pharmacy Affairs (OPA) › Administrates over the 340B Drug-Pricing Program › Develops innovative pharmacy service models and provides technical assistance to help entities implement effective pharmacy programs › Serves as a federal resource about pharmacy › Emphasizes the importance of comprehensive pharmacy services functioning as integral part of primary health care Integrity Prime Vendor Program(PVP) › Relationships and networking › Policy analysis › Education o 340B University › Technical assistance o Apexus Answers Call center o 340B tools and resources o www.340bpvp.com Access Prime Vendor Program (PVP) › Negotiation of o 340B sub-ceiling pricing o Discounts on value-added products, services, and supplies › Overcharge recovery › Pricing transparency › Reports and tools › Technical assistance Value 340B Resource Information Health Resources and Services Administration http://www.hrsa.gov/opa/ http://www.hrsa.gov/publichealth/clinical/patientsafety/index.html 340B Prime Vendor Program Managed by Apexus 1-888-340-2787 ApexusAnswers@340bpvp.com https://www.340bpvp.com/ Thank you for viewing this 340B tutorial developed by : Health Resources and Services Administration Office of Pharmacy Affairs 340B Peer-to-Peer Program You can view additional 340B educational products and tools specifically developed to assist 340B-participating entities create and maintain processes to ensure 340B program integrity at: www.hrsa.gov/opa/peertopeer/