PowerPoint - 埼玉医科大学総合医療センター 内分泌・糖尿病内科
advertisement

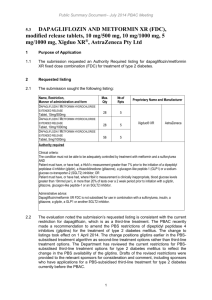
Journal Club Dziuba J, Alperin P, Racketa J, Iloeje U, Goswami D, Hardy E, Perlstein I, Grossman HL, Cohen M. Modeling effects of SGLT-2 inhibitor dapagliflozin treatment versus standard diabetes therapy on cardiovascular and microvascular outcomes. Diabetes Obes Metab. 2014 Jul;16(7):628-35. 2014年8月28日 8:30-8:55 8階 医局 埼玉医科大学 総合医療センター 内分泌・糖尿病内科 Department of Endocrinology and Diabetes, Saitama Medical Center, Saitama Medical University 松田 昌文 Matsuda, Masafumi Diabetologia 55:1577-96, 2012, Diabetes Care 35:1364-1379, 2012 Aims Dapagliflozin, a sodium-glucose cotransporter 2 (SGLT-2) inhibitor, has been shown to lower glycated hemoglobin (HbA1c), weight, blood pressure and serum uric acid in clinical trials. Plasma lipids were also evaluated as exploratory variables. The goal of this study was to estimate the long-term cardiovascular (CV) and microvascular outcomes of dapagliflozin added to the standard of care (SOC) versus SOC using simulation methodology. Methods The Archimedes Model, a validated model of human physiology, diseases and healthcare systems, was used to model a type 2 diabetes mellitus (T2DM) population derived from National Health and Nutrition Examination Survey (NHANES) with HbA1c 7–10%, taking a single oral antidiabetic agent [metformin, sulfonylureas SU or thiazolidinedione (TZD)] at the beginning of the trial. A 20-year trial was simulated comparing dapagliflozin 10 mg, given in addition to SOC, with SOC alone. SOC was based on American Diabetes Association (ADA)/European Association for the Study of Diabetes (EASD) 2012 guidelines and included diet, metformin, SU, TZD, dipeptidyl peptidase-4 (DPP-4), glucagon-like peptide-1 (GLP1), and insulin therapies, with usage levels reflective of those in NHANES. Dapagliflozin effects were derived from phase 3 clinical trial results. End points included CV and microvascular outcomes. We evaluated dapagliflozin as an add-on therapy in patients with T2DM uncontrolled on established DM oral monotherapy treatment [i.e. metformin, sulfonylureas (SU), or thiazolidinedione (TZD)]. We created a simulated population of 30 000 per treatment strategy by using person-specific data from the NHANES (years 1999–2008). A possible consequence of this could be an overestimation of benefit from SGLT2 inhibitors due to a lower than expected usage of statins and/or glucagon-like peptide-1 (GLP1)/dipeptidyl peptidase-4 (DPP-4) therapies. The first, dapagliflozin-1, included the glucose-, BP-, weight- and SUA-lowering effects associated with dapagliflozin. The second, dapagliflozin-2, included these effects plus effects on lipids. Because we aimed to evaluate dapagliflozin as an add-on therapy to established T2DM monotherapy treatments, our efficacy assumptions (Table 1) were based on pooled data from phase III clinical trials that evaluated dapagliflozin as an add-on treatment to metformin, SU and TZD, including four studies that assessed HbA1c, weight, SUA and the lipid panel [7-10]. Assumptions for BP change were based on results from two add-ons to usual care studies that assessed systolic BP(SBP) as a key secondary end point [11, 12]. In all treatment strategies the trial protocol began with an initial visit at the start of the simulated trial. Patients included in the study were uncontrolled on T2DM monotherapy treatment. In the SOC arm, patients were prescribed second-line SOC diabetes treatments. In the dapagliflozin arms, the patients were prescribed dapagliflozin. In all arms, a follow-up visit was scheduled in 3 months. At this visit and with subsequent follow-up care additional treatments were prescribed to patients with HbA1c > 7.0% (uncontrolled) according to the 2012 ADA/EASD guidelines. Follow-up visits were scheduled every 3 months if uncontrolled and every 6 months if controlled. 5.3mM=205mg/dl T-chol 5mM=193mg/dl 1.3mM=50mg/dl 1.1mM=42mg/dl 2.65mM=235mg/dl TG 2.25mM=199mg/dl HDL-C 3mM=116mg/dl 2.75mM=106mg/dl LDL-C SOC Dapagliflozin-1 Dapagliflozin-2 Figure 4. Number needed to treat with dapagliflozin-1 and dapagliflozin-2 treatments to avoid an outcome relative to standard of care (SOC) at 20 years among uncontrolled type 2 diabetes mellitus (T2DM) cohort. [MACE, major adverse cardiovascular events (fatal and non-fatal MI, fatal and nonfatal stroke along with CV death), expanded MACE, MACE and foot amputation; MI, myocardial infarction; CV, cardiovascular; ESRD, end-stage renal disease]. Results Over a 20-year period, patients on dapagliflozin were projected to experience relative reductions in the incidence of myocardial infarction (MI), stroke, CV death, and all-cause death of 13.8, 9.1, 9.6 and 5.0%, respectively, and relative reductions in the incidence of end-stage renal disease (ESRD), foot amputation, and diabetic retinopathy of 18.7, 13.0 and 9.8%, respectively, when compared with SOC. Conclusions On the basis of simulation results, adding dapagliflozin to currently available treatment options is projected to further decrease the CV and microvascular complications associated with T2DM. Message SGLT2阻害薬をこれまでの標準的な治療に追加で 用いると血管合併症の低下が予想されるという 結果である。 副作用やドロップアウトについては? 実際の薬の使い方のウェイトにより結果は変わ ると思われるので、この解析はどのくらい現実 をシミュレートできているのか疑問。特に日本 ではDPP-4阻害薬をもっと使うのでかなり現実と 異なっていると感じる。