GI ARS model - Advances in Inflammatory Bowel Diseases
advertisement
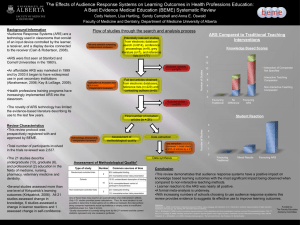
Modulation of inflammatory processes in DSS-induced colitis model versus gastrointestinal acute radiation syndrome with AVX-470m, an oral polyclonal anti-TNF antibody Kailash C Bhol, Ph.D, Brenda Lemos, B.S, Emma Erlich, B.A., David Keane, B.S, Daniel E. Tracey, Ph.D., Barbara S. Fox, Ph.D., and Deborah Hartman, Ph.D. CCFA 2013 1 Disclosures • Employee of Avaxia Biologics, Inc. 2 Introduction • AVX-470 is a gut-targeted polyclonal anti-TNF antibody that is orally delivered and acts locally in the GI tract1 • AVX-470 is being developed for inflammatory bowel disease (IBD), and for gastrointestinal acute radiation syndrome (GI ARS) as a secondary indication • Designed to overcome the limitations of current injectable anti-TNF therapies – direct delivery to the site of inflammation – minimal systemic exposure for reduced side effects from untargeted immunosuppression – potential for prolonged duration of effect by avoiding neutralizing anti-drug antibodies 3 1Bhol et al, Inflamm Bowel Dis. 2013 Oct;19(11):2273-81 Background • AVX-470m is a murine-specific surrogate antibody1 – Potent and selective, neutralizes mouse TNF – Effective in DSS- and TNBS- induced colitis models • TNF is strongly implicated in GI ARS, which results from exposure to high doses of ionizing radiation – GI ARS results from a nuclear accident, explosion, accidental release or act of terrorism; devastating consequences in GI tract driven by cytokine-mediated inflammation closely resembling the pathophysiology of IBD • In this study, we have profiled AVX-470m effects on biomarkers of inflammation in IBD and GI ARS models. 4 1Bhol et al, Inflamm Bowel Dis. 2013 Oct;19(11):2273-81 GI ARS: structural damage and mortality curve AVX-470m treatment showed survival benefit in the GI ARS model, compared to saline and control Ig. 5 DSS-induced colitis vs GI ARS: Induction of inflammatory biomarkers Response to injury • • • 6 Biomarker DSS-induced colitis (colon) GI ARS (jejunum) TNF TNFR1 * ↑ ↑ ↑ ─ TNFR2 * ↑ ↑ MPO ↑ ↑ MMP9 * CD3 CD68 ↑ ↑ ↑ ↑ ─ ─ IL-1β ↑ ↑ IL-12p40 ↑ ─ ICAM-1 * ↑ ↑ ↑ indicates: Significant increase over control in DSS colitis (p<0.05) Or, > 2-fold increase in GI ARS Biomarkers measured by immunohistochemistry, and qPCR (*) Colitis: C57BL/6 mice with 3% DSS in drinking water for five days GI ARS: C57BL/6 mice irradiated with 15.9 Gy, partial bone marrow shielding, 60Co gamma radiation source Treatment with AVX-470m reduces TNF levels in both IBD and GI ARS models IBD - colon tissue sections No DSS DSS + Saline DSS + AVX-470m 15.9 Gy + Saline 15.9 Gy + AVX-470m GI ARS - jejunum tissue sections No Radiation • 7 AVX-470m (10 mg/day, BID by oral gavage) reduced TNF protein levels in GI tissue in both IBD and GI ARS models AVX-470m treatment reduces inflammatory biomarkers in both IBD & GI ARS models Response to injury DSS-induced colitis (colon) TNF ↑ TNFR1* ↑ TNFR2* ↑ MPO ↑ MMP9* ↑ CD3 ↑ CD68 ↑ IL-1B ↑ IL-12p40 ↑ ICAM-1 (qPCR) ↑ Biomarker GI ARS (jejunum) ↑ ─ ↑ ↑ ↑ ─ ─ ↑ ─ ↑ + AVX-470m DSS-induced GI ARS colitis (colon) (jejunum) ↓ ↓ ─ ─ ─ ─ ↓ ↓ ↓ ↓ ─ ─ ↓ ─ ↓ ─ ↓ ─ ─ ─ ↓ indicates p<0.05 for IBD markers or > 2-fold decrease for GI ARS markers. Control Ig had no effect on inflammatory biomarkers. *= qPCR analysis. 8 AVX-470m penetrates GI mucosal barrier Tissue sections stained for presence of bovine Ig Normal Control animal with AVX-470m • • 9 IBD model - colon DSS with AVX-470m GI ARS model - jejunum 15.9 Gy with AVX-470m AVX-470m penetrates into damaged tissue in both colon and jejunum, but not healthy tissue. Labeling is seen in mucosa, lamina propria, and submucosal regions. Minimal systemic exposure is seen with AVX-470m Conclusions • DSS colitis and GI ARS share a common TNFmediated inflammatory component • AVX-470m is a novel gut-targeted anti-TNF antibody that is delivered orally and acts locally in the GI tract • Oral delivery of AVX-470m effectively inhibits TNF expression in DSS colitis and GI ARS • These data support the therapeutic potential of an oral anti-TNF antibody as a gut-targeted treatment for multiple GI inflammatory diseases in both the large and small bowel. • AVX-470 is in Phase 1b clinical trials in patients with active ulcerative colitis, results in early 2014. 10 Acknowledgments • Avaxia Biologics, Inc. – – – – – – Barbara Fox, PhD Dan Tracey, PhD Kailash Bhol, PhD Brenda Lemos Emma Erlich Dave Keane DSS-induced colitis model • Biomodels, LLC – Steve Sonis, DMD, DMSc – Greg Lyng, PhD GI ARS model • CiToxLAB North America – Simon Authier, PhD This project has been funded in part by the Biomedical Advanced Research and Development Authority (BARDA), Office of the Assistant Secretary for Preparedness and Response, Department of Health and Human Services under Contract No. HHSO100201100027C. This work has also been supported in part by in part by National Institutes of Health (NIH) grant 2R44DK083810-02 11