Ballantyne Plenary Panel 2014
advertisement
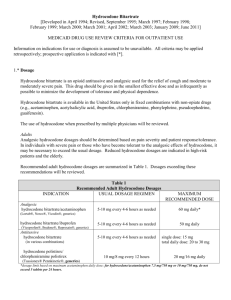
Legislation-based solutions Jane C Ballantyne, University of Washington, Seattle How pain treatment became mandated in the US “No one who thinks of the early nineteenth-century opium addicts in terms of what their position would be today – forced to pester reluctant doctors …. or to pay large sums for illicit supplies … – will be able to understand the frame of mind of someone like Coleridge, who had no obstacles between him and the drug but his own conscience and the reproaches of his immediate family and closest friends …… Alethea Hayter in Opium and the Romantic Imagination Twentieth Century American War on Drugs 1910 Foster Act Controlled opiates, cocaine, chloral and cannabis. Failed in 1911. 1914 Harrison Act Required registration and payment of an occupational tax by all those who imported, produced, dealt in, sold or gave away opium and coca leaves and their derivatives. Remained the foundation of controls on narcotics until 1970. 1919 Webb versus the US Prohibited physicians from dispensing maintenance prescriptions to people with cravings. Immediate switch of moral imperative from user to prescriber • Early 20th century regulations led to gross undertreatment of both pain and addiction in the US • This began to be corrected in the mid-20th century • Laws were written to protect prescribers Institution of opioid maintenance treatment for addiction in the US 1974 Narcotic Addict Treatment Act 1993 Approval of LAAM (l-methadyl acetate) 1999 Proposal to adopt new regulations with provision for office-based treatment 2000 Drug Addiction Treatment Act • “I would rather be in pain than be considered an addict” • by the mid 20th Century, there seemed an ethical basis for compelling opioid treatment of pain (relieve pain & suffering) and no ethical basis for withholding treatment (no risk of addiction) Protecting clinicians when prescribing for pain 1997 Intractable pain policies 1997 Federation of State Medical Board Policy Statement 2000 DEA Policy Statement Gilson et al Health Policy 2005;74:192-204 Joranson & Gilson APB Bulletin 1997;7:7-9 West et al Federation of State Medical Boards 1998 DEA Frequently asked questions 2005 Current quandary for the US Institute of Medicine (IOM) Report In the committee’s view, addressing the nation’s enormous burden of pain will require a cultural transformation in the way pain is understood, assessed, and treated. This report provides recommendations intended to help achieve this transformation Medicine and public health measures have succeeded in greatly increasing longevity. But although we live longer as a population, we do not necessarily live better. A 2011 congressionally mandated study by the Institute of Medicine Committee on Advancing Pain Research, Care, and Education reported that 116 million Americans suffer from chronic pain, costing up to $635 billion annually in treatment and lost productivity http://www.nap.edu 2001 Pain management became mandated by US accreditation body • Mandate required that pain be recognized, assessed, documented and treated • Also required that systems be in place to achieve these goals • The use of the “fifth vital sign” became instantly popular JCAHO Pain management standards 2001 Importance of patient satisfaction as a quality metric in US Healthcare • Used for quality improvement as well as benchmarking • Attention to pain, and successful treatment of pain have become important quality metrics • Satisfaction rating are used not only for the accreditation of healthcare facilities, but also to assess the performance of individual clinics and individual clinicians • Failure to comply could mean loss of institutional support, institutional failure or loss of livelihood for individual clinicians • Instruments used to measure patient satisfaction are notoriously unreliable Ballantyne & Fleisher PAIN 2010;148:365-7 Zgierska et al JAMA 2012;307:1377-8 Efforts to swing the pendulum back have become highly political 1. Washington State passes law restricting opioid dosing (2011) 2. Citizen’s petition presented to Food and Drug Administration (FDA) requesting radical relabeling of opioids when used for chronic pain (2012) 3. Senate enquiry instigated regarding FDA’s approval of long-acting hydrocodone, against panel’s advice (2014) Rule 2876 2010 1. Sets standards to use when considering state licensing disciplinary action against providers 2. Rules directed only to Chronic Opioid Treatment of Chronic Non-Cancer Pain 3. Requires full H & P 4. Requires 4 hrs pain CME 5. Dose criteria above which provider must consult with a pain specialist at > 120mg MED 6. Not needed if function improved, dose stable or tapering, no special risk, provider meets certain qualifying criteria Fears brought about by the Washington State Law • Access to pain medications will be restricted • Clinicians will stop prescribing • Pain will become undertreated again • Patients will be abandoned • Laws should not interfere with clinical decision making What actually happened Good • • • • Provided an opportunity to educate Necessitated development of telemedicine Death rates have gone down Scale of the problem of opioid dependence exposed Bad • Patients have been abandoned • Some practices have instituted a “no opioid” policy • Dependent patients who have been cut off are turning to heroin Franklin et al Am J Ind Med 2012;51:325-31 What are the current problems with Rule 2876 NOT EASY TO FIX • There is no consensus about what to do with the people already on high doses, even among experts • There are no suitable services for people with dependence on opioid pain medications “Complex persistent dependence” • taper or maintain? • strong evidence that patients who taper from high doses improve in terms of general function and well-being • unfortunately many relapse • tapering is hard to achieve for all but the most motivated • rehab setting is more successful than outpatient setting • need pain treatment as well as dependence treatment Ballantyne & LaForge Pain 2007;129:235-55 Ballantyne & Sullivan Arch Intern Med 2012;172:1242-3 What the Washington State Law has taught 1. There are a lot of individuals whose lives have been decimated by overuse of opioids 2. These individuals need specialty care, which often is not available 3. In future, opioid prescribing should be much more selective in terms of who gets treated, for how long and at what dose 1. Source: Physicians for Responsible Opioid Prescribing http://www.supportprop.org/educa tional/PROP_OpioidPrescribing.pdf Citizen’s petition calls for the following changes on opioid labels 1. Strike the term “moderate” from the indication for non-cancer pain. 2. Add a suggested maximum daily dose, equivalent to 100 milligrams of morphine for non-cancer pain. 3. Add a suggested maximum duration of 90-days for continuous (daily) use for non-cancer pain. Zohydro – New long acting hydrocodone • Reviewed by FDA Advisory Committee on December 2012 • Advisory Committee votes 11-2 against approval • Approved by FDA October 2013 How the Opioid Industry Frames the Problem: Source: Slide presented at FDA meeting on hydrocodone up-scheduling, January 25th, 2013. Senators Question FDA’s Approval of Powerful Painkiller The Huffington Post WASHINGTON (AP) — Three U.S. senators are raising concerns about the Food and Drug Administration's approval of a powerful painkiller called Zohydro, which experts say could add to the national epidemic of prescription drug abuse. Republicans Mitch McConnell of Kentucky, Tom Coburn of Oklahoma and Lamar Alexander of Tennessee sent a letter to the head of the FDA Wednesday asking how the agency will prevent misuse and abuse of Zohydro and similar drugs in development. The FDA approved Zohydro from Zogenix Inc. in October, making it the first singleingredient hydrocodone drug ever cleared for U.S. patients. The pill is significantly more potent than currently available hydrocodone combination pills, such as Vicodin. The approval surprised many doctors, since an FDA advisory panel voted overwhelmingly against the drug, citing its potential for abuse. What happened in the US 1. Harrison Act in 1914 switched the moral burden to clinicians and Webb vs US in 1918 made US clinicians especially fearful of prescribing even for pain 2. The efforts of pain advocates in the 1980s made opioids more available 3. Pain management in healthcare facilities became mandated and the “fifth vital sign” was used as a means of demonstrating attention to pain (2001) 4. Opioid use skyrocketed and so did opioid abuse. Characterized as an ‘epidemic’ by the CDC in 2012 5. Affordable Care Act instituted IOM evaluation of the problem of uncontrolled pain 6. Laws enacted to try and control opioid overuse 7. Political means used to try and expose activities of the pharmaceutical industry in overpromoting opioids REFERENCES 1. IOM (Institute of Medicine). Relieving Pain in America. A Blueprint for Transforming Prevention, Care, Education, and Research. The National Academies Press, Washington, DC. http://www.nap.edu. 2011. 2. CDC Grand Rounds: Prescription Drug Overdoses - a US Epidemic. 61(01):1013 2012;http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6101a3.htm. 3. Ethics charter from American Academy of Pain Medicine. Pain Med 2005;6:203-12. 4. Carr DB, Goudas LC. Acute pain. Lancet 1999;353:2051-8. 5. Kehlet H, Dahl JB. Anaesthesia, surgery, and challenges in postoperative recovery. Lancet 2003;362:1921-8. 6. Brennan F, Carr DB, Cousins M. Pain management: a fundamental human right. Anesth Analg 2007;105:205-21. 7. Medina JL, Diamond S. Drug dependency in patients with chronic headache. Headache 1977;17:12-4. 8. Porter J, Jick H. Addiction rare in patients treated with narcotics (letter). N Engl J Med 1980;302:123. 9. Portenoy RK, Foley KM. Chronic use of opioid analgesics in non-malignant pain: report of 38 cases. Pain 1986;25:171-86. 10. Brennan TA. Just Doctoring. Medical Ethics in the Liberal State. University of California Press, Berkeley and Los Angeles, CA. 1991:p 3 (Brennan quotes the Webster Dictionary definition of liberalism). 11. Dubois MY. The birth of an ethics charter for pain medicine. Pain Med 2005;6:2012. 12. Dubois MY, Gallagher RM, Lippe PM. Pain medicine position paper. Pain Med 2009;10:972-1000. 13. Joranson DE, Gilson AM. State intractable pain policy: Current status. APS Bulletin 1997;7:7-9. 14. Joranson DE, Gilson AM, Dahl JL, Haddox JD. Pain medicine, controlled substances, and state medical board policy: a decade of change. J Pain Symptom Manage 2002;23:138-47. 15. Advisory Commission on Consumer Protection and Quality in the Health Care Industry. Patients' Rights and Responsibilities http://www.consumer.gov/qualityhealth/rights/htm . 1998. 16. Joint Commission on Accreditation of Healthcare Organizations Pain management standards. Effective January 1, 2001. Available at: http://www.jcaho.org/standard/. 2001. 17. Ballantyne JC, Fleisher LA. Ethical issues in opioid prescribing for chronic pain. Pain 2010;148:365-7. 18. Zgierska A, Miller M, Rabago D. Patient satisfaction, prescription drug abuse, and potential unintended consequences. JAMA 2012;307:1377-8. 19. Washington State Department of Health, Health Systems Quality Assurance, Pain Management Adopted Rules, at http://wwwdohwagov/hsqa/professions/painmanagement/meetingshtm 2012. 20. Ballantyne JC. Clinical and administrative data review presented to FDA May 30th and 31st 2012. 2012. 21. Morasco BJ, Duckart JP, Carr TP, Deyo RA, Dobscha SK. Clinical characteristics of veterans prescribed high doses of opioid medications for chronic non-cancer pain. Pain 2010;151:625-32. 22. Edlund MJ, Martin BC, Fan MY, Devries A, Braden JB, Sullivan MD. Risks for opioid abuse and dependence among recipients of chronic opioid therapy: results from the TROUP study. Drug Alcohol Depend 2010;112:90-8. 23. Weisner CM, Campbell CI, Ray GT, et al. Trends in prescribed opioid therapy for noncancer pain for individuals with prior substance use disorders. Pain 2009;145:287-93. 24. Turk DC, Okifuji A. Pain terms and taxonomies. In: Fishman, SM, Ballantyne JC, Rathmell JP eds Bonica's Management of Pain (4th ed) Lippincott Williams and Wilkins pp 14-23. 2010. 25. Braden JB, Sullivan MD, Ray GT, et al. Trends in long-term opioid therapy for noncancer pain among persons with a history of depression. Gen Hosp Psychiatry 2009;31:564-70. 26. Korff MV, Saunders K, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain. Clin J Pain 2008;24:521-7. 27. Martin BC, Fan MY, Edlund MJ, Devries A, Braden JB, Sullivan MD. Long-term chronic opioid therapy discontinuation rates from the TROUP study. J Gen Intern Med 2011;26:1450-7. 28. Volinn E, Fargo JD, Fine PG. Opioid therapy for nonspecific low back pain and the outcome of chronic work loss. Pain 2009;142:194-201.