Electron transport chain - Ms. Springstroh Lane Tech AP Biology
advertisement

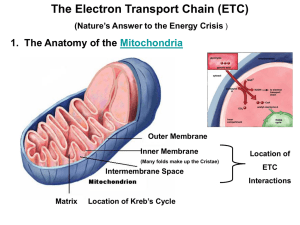
Cellular Respiration: Electron Transport Chain Ch. 9 Ms. Springstroh AP Biology Adapted from Ms. GaynorDay and Mr. Grant What’s the point? The point is to make ATP! ATP AP Biology 2006-2007 ATP accounting so far… Glycolysis 2 ATP Kreb’s cycle 2 ATP Life takes a lot of energy to run, need to extract more energy than 4 ATP! There’s got to be a better way! I need a lot more ATP! AP Biology A working muscle recycles over 10 million ATPs per second Stage #3: Oxidative Phosphorylation (Electron Transport Chain (ETC) + Chemiosmosis) Chemiosmosis is a process which connects the processes of electron transport and ATP synthesis NADH and FADH2 Drop off e-’s at ETC, which powers ATP synthesis using oxidative phosphorylation **OCUURS IN CRISTAE (folds of inner membrane) Mitochondria Double membrane outer membrane inner membrane highly folded cristae enzymes & transport proteins intermembrane space fluid-filled space between membranes Oooooh! Form fits function! What is “oxidative phosphorylation”? Recall… H+/e-’s away, molecule = “oxidized” Give H+/e-’s, molecule = “reduced” Give phosphate molecule = “phosphorylated” Take So…oxidative phosphorylation = process that couples removal of H+’s/ e-’s from one molecule (NADH or FADH2) & giving phosphate molecules to another molecule (ADP) Difference between oxidative phosphorylation & substrate-level phosphorylation Oxidative phosphorylation: generates ATP when electrons are taken from NADH or FADH2 (which become oxidized) and go down the electron transport chain. This causes Pi (inorganic phosphate) to join with ADP to form ATP. Substrate-level phosphorylation: generates ATP when an enzyme takes a phosphate from a substrate molecule and gives it directly to ADP. The Pathway of Electron Transport In the ETC… fall from glucose to oxygen not directly, rather in a series of steps. As the e-’s fall from step to step, energy e-’s is released in manageable amounts. **NEEDS O2 TO PROCEED (unlike glycolysis) ETC Characteristics Occurs in cristae, which increase surface area of inner mitochondrial membrane allows more ATP to be produced ETC takes e-’s from NADH/FADH2 and gives them to O2 O2 “pulls” e-’s “down” ETC due to electronegativity (high affinity for e-’s) What happens at the end of the ETC chain? Electrons are passed to oxygen, forming water O 2 = final e- acceptor How ETC generates ATP ETC does NOT make ATP directly but provides the stage for chemiosmosis to occur energy from “falling” e-’s (exergonic) in the ETC is used to pump H+’s from mitochondrial matrix to intermembrane space Results in a H+ (proton) gradient inside mitochondria The Inside (matrix) = low [H+] Outside (intermembrane space) = high [H+] http://highered.mcgrawhill.com/olcweb/cgi/pluginpop.cgi?it=swf::53 5::535::/sites/dl/free/0072437316/120071/bi o11.swf::Electron%20Transport%20System %20and%20ATP%20Synthesis Oxidative phosphorylation. electron transport and chemiosmosis Glycolysis ATP ATP Inner Mitochondrial membrane ATP H+ H+ Chemiosmosis and the electron transport Protein complex chain of electron H+ H+ Cyt c Intermembrane space carriers Q I IV III ATP synthase II Inner mitochondrial membrane FADH2 NADH+ FAD+ 2 H+ + 1/2 O2 NAD+ ADP + (Carrying electrons from, food) Mitochondrial matrix ATP Pi H+ Electron transport chain (H+), Electron transport and pumping of protons which create an H+ gradient across the membrane Figure 9.15 H2O Chemiosmosis ATP synthesis powered by the flow Of H+ back across the membrane Oxidative phosphorylation Chemiosmosis A mechanism which uses energy stored in the H+ gradient across any membrane to drive cellular work Cellular work in this case = synthesis of ATP Utilizes ATP synthase the enzyme that actually makes ATP from ADP and Pi Chemiosmosis: The EnergyCoupling Mechanism INTERMEMBRANE SPACE H+ H+ H+ H+ H+ H+ H+ H+ ATP Synthase Figure 9.14 ADP + Pi MITOCHONDRIAL MATRIX ATP Electron shuttles span membrane CYTOSOL MITOCHONDRION 2 NADH or 2 FADH2 2 NADH 2 NADH 6 NADH 2 FADH2 There are three main processes inOxidative this phosphorylation: metabolic enterprise electron transport Glycolysis Glucose 2 Pyruvate + 2 ATP by substrate-level phosphorylation Maximum per glucose: 2 Acetyl CoA Citric acid cycle + 2 ATP by substrate-level phosphorylation About 36 or 38 ATP and chemiosmosis + about 32 or 34 ATP by oxidative phosphorylation We will cover the following two slides when we learn about photosynthesis, but you can preview them now if you want. You’ll probably understand most of them! A Comparison of Chemiosmosis in Chloroplasts and Mitochondria Chloroplasts and mitochondria ATP by the SAME basic mechanism: chemiosmosis But use different sources of energy to accomplish this Generate http://student.ccbcmd.edu/~gkaiser/biotutorials/cellresp/atp ase_flash.html The spatial organization of chemiosmosis differs in chloroplasts and mitochondria In both organelles electron transport chains generate a H+ gradient across a membrane ATP synthase Uses this protonmotive force to make ATP