08_29_30 HW Notes
advertisement
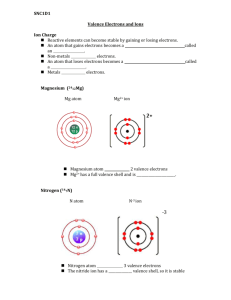
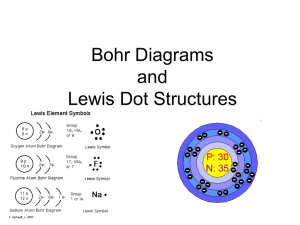
Chemistry Homework Notes 08.30.13 Read pp. 158-165, problems pp. 165 #1-5, 10 1. Explain why the noble gases tend not to react. Noble gases tend not to react because they have a full valence (outer) energy level of electrons. (pp. 158-159.) 2. Where are the valence electrons located in an atom? The valence electrons are located in the outer (farthest from the nucleus) energy level. (p. 160 & Figure 3.) 3. How does a cation differ from an anion? Cations are positively charged ions and anions are negatively charged ions. A cation is formed when an atom loses one or more electrons. An anion is formed when an atom gains one or more electrons. (p. 161 & Figure 4.) 4. State the octet rule. The octet rule states that atoms tend to gain or lose electrons to form the same electron configuration as a noble gas. (p. 159, 161.) 5. Why do the properties of an ion differ from those of its parent atom? The properties of an ion are different from the properties of the parent atom because the ion has either gained or lost electrons to become an ion. The ion has a different number of electrons and different electron configuration than the parent atom. Because electrons determine chemical properties, changing the electrons changes the properties of the substance. (p. 162.) 10. How could each of the following atoms react to achieve a noble gas configuration? A. iodine – This is a halogen in group 17. It has 7 valence electrons and will gain 1 to form a stable ion. B. strontium – This is a alkaline earth metal in group 2. It has 2 valence electrons. It will lose 2 electrons to form a stable ion. C. Nitrogen – Group 15 non-metal. It has 5 v.e. It will gain 3 to form a stable ion. D. Krypton – Noble gas. No reaction.