Chapter 6 - Bonding in Metals
advertisement
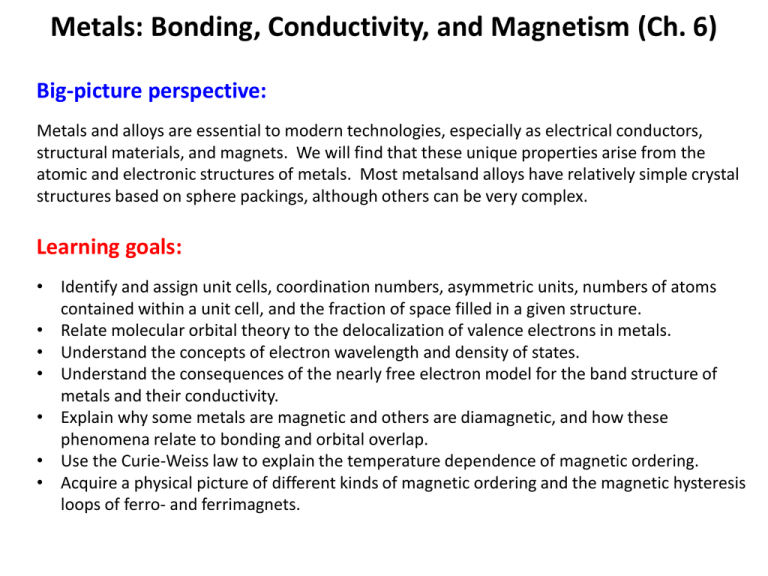
Metals: Bonding, Conductivity, and Magnetism (Ch. 6) Big-picture perspective: Metals and alloys are essential to modern technologies, especially as electrical conductors, structural materials, and magnets. We will find that these unique properties arise from the atomic and electronic structures of metals. Most metalsand alloys have relatively simple crystal structures based on sphere packings, although others can be very complex. Learning goals: • Identify and assign unit cells, coordination numbers, asymmetric units, numbers of atoms contained within a unit cell, and the fraction of space filled in a given structure. • Relate molecular orbital theory to the delocalization of valence electrons in metals. • Understand the concepts of electron wavelength and density of states. • Understand the consequences of the nearly free electron model for the band structure of metals and their conductivity. • Explain why some metals are magnetic and others are diamagnetic, and how these phenomena relate to bonding and orbital overlap. • Use the Curie-Weiss law to explain the temperature dependence of magnetic ordering. • Acquire a physical picture of different kinds of magnetic ordering and the magnetic hysteresis loops of ferro- and ferrimagnets. 2/3 of the elements are metals Unit cells Parallelepiped from which the entire crystal can be built up by purely translational displacements Unit cells Unit cells Unit cells Unit cells Unit cells and lattices Unit cells and lattices We can generate the “2D” NaCl structure by placing the “NaCl” asymmetric unit (basis) on each lattice point of a cubic lattice 14 Bravais lattices lattice points onto which asymmetric units are placed Body centered cubic (bcc) structure How many complete atoms in the unit cell? Coordination number? Close-packed structures Simple cubic and body centered cubic do not maximize the filling of space. Think about the best way to fill space with hard spheres… Hexagonal vs. cubic close packed hcp ccp (= fcc) Cubic close packed (ccp) is face centered cubic (fcc) Face centered cubic (fcc) structure How many complete atoms in the unit cell? Coordination number? Work on this together in groups Crystal structures of the elements Bonding and Conductivity What are the periodic trends? Molecular Orbitals in Metals 1-D Chain of Na atoms What is the wavelength of an electron in these MO's? Molecular Orbitals in Metals Infinite chain of Na atoms What are the energies of the MO's in metals? Molecular Orbitals in Metals What are the energies of the MO's in metals? Nearly free electron model: KE = ½ mv2 = p2/2m = h2/2mλ2 k = π/a h2k2 E = 8π2m … 2D 3D … Energy … 1D EF … k (= 2π/λ) Density of States (Number of orbitals per unit energy) Band Diagrams Metals vs. Insulators (or Semiconductors) Conduction in Metals Metals vs. Insulators (or Semiconductors) "Nearly free" electrons conduct electricity and heat Conduction in Metals • Electrons in metals are accelerated by an electric field, but they scatter by interacting with the lattice (lattice vibrations, defects, impurities) • The mean free path is long (~40 nm) compared to the atomic spacing (0.2 nm) (football field vs. football). Thus we have an electron "gas" • Scattering gives rise to resistance (Ohm's law, V = iR) Bonding, Energetics, and Magnetism Why is Mg (=[Ne]3s2) a metal? The promotion energy (3s2 3s13p1) is less than the bonding energy Bonding, Energetics, and Magnetism • How many bonding electrons does each atom have? • Why does W have such strong bonding? • Why are the 3d elements different from 4d & 5d? Bonding, Energetics, and Magnetism Which transition metals are magnetic? Four kinds of magnetic behavior } } } Other kinds of spin ordering: Antiferromagnetic Ferrimagnetic No unpaired spins Spin alignment in a magnetic field Paramagnets follow Curie Law behavior c= low T C T high T Spins align in a magnetic field at low T No ordering in the absence of an applied field Ferro-, ferri-, and antiferromagnets Above Tc, ferro/ferrimagnets follow the Curie-Weiss Law c= C T - TC Spins spontaneously order below TC Paramagnetic behavior above TC Antiferromagnets resist parallel alignment of spins => negative TC C c= T +q Also paramagnetic above TC Ferro-/ferrimagnets below TC What competing energies cause magnets to have micron-size domains? How do domain walls move in an applied magnetic field? Magnetic hysteresis loops What are hard vs. soft magnets? When would you want a soft magnet?