Refrigeration Cycle
advertisement
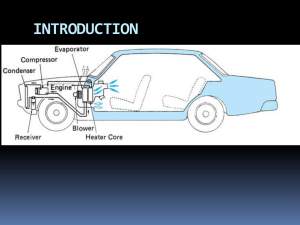
Thermodynamics Gas Power Cycles Gas Power Cycles Otto Cycle Single Cylinder Four Stroke Spark Ignition Engine Multi-Cylinder Spark Ignition Engine THE OTTO CYCLE COMPRESSION STROKE Air and fuel are mixed and compressed so rapidly that there is no time for heat to be lost. (Figure A) In other words the compression is adiabatic. Work must be done to compress the gas. IGNITION Just before the point of maximum compression, the air is hot and a spark ignites the mixture causing an explosion (Figure B). This produces a rapid rise in the pressure and temperature. The process is idealized as a constant volume process in the Otto cycle. EXPANSION OR WORKING STROKE The explosion is followed by an adiabatic expansion pushing the piston and giving out work. (Figure C) EXHAUST At the end of the working stroke, there is still some pressure in the cylinder. This is released suddenly by the opening of an exhaust valve. (Figure D) This is idealized by a constant volume drop in pressure in the Otto cycle. In 4 stroke engines a second cycle is performed to push out the products of combustion and draw in fresh air and fuel. It is only the power cycle that we are concerned with The four ideal processes that make up the Otto cycle are as follows. The Ideal Cycle for Spark Ignition Engine ( the Otto Cycle ) Assumptions: - The working medium is a thermally perfect gas with constant specific heats ( taken as those of air at atmospheric conditions) - The compression and expansion strokes are isentropic- Heat addition from external source and heat rejection to external sink and both take place at constant volume WORKED EXAMPLE : An Otto cycle is conducted as follows. Air at 100 kPa and 20oC is compressed reversibly and adiabatically. The air is then heated at constant volume to 1500oC. The air then expands reversibly and adiabatically back to the original volume and is cooled at constant volume back to the original pressure and temperature. The volume compression ratio is 8. Calculate the following. i. The thermal efficiency. ii. The heat input per kg of air. iii. The net work output per kg of air. iv. The maximum cycle pressure. cv = 718 kJ/kg K R = 287 J/kg K SOLUTION Remember to use absolute temperatures throughout. Solve for a mass of 1 kg. T1=20 +273=293K T3=1500+273=1773K rc=8 Diesel – Engine The gasoline-engine (Otto-engine) wasn't very efficient, that is why 1892 Rudolf Diesel had developed the engine with great efficiency named after him. It is like the Otto-engine a 4-stroke engine. The main differences are: 1st stroke (Intake): Only air is sucked in. 2nd stroke (Compression): The air is powerfully compressed. 3rd stroke (Combustion): Diesel is directly injected into the compressed air and ignites spontaneously. 4th stroke (Exhaust): Like the Otto-engine DUAL COMBUSTION CYCLE This is the air standard cycle for a modern fast running diesel engine. First the air is compressed isentropically making it hot. Fuel injection starts before the point of maximum compression. After a short delay in which fuel accumulates in the cylinder, the fuel warms up to the air temperature and detonates causing a sudden rise in pressure. This is ideally a constant volume heating process. Further injection keeps the fuel burning as the volume increases and produces a constant pressure heating process. After cut off, the hot air expands isentropically At the end of the stroke, the exhaust valve opens producing a sudden drop in pressure. This is ideally a constant volume cooling process. The ideal cycle is shown in figure The analysis of the cycle is as follows. The heat is supplied in two stages hence Qin = mcp(T4 - T3) + mcv (T3 - T2) The heat rejected is Qout = mcv (T5 - T1) The thermal efficiency may be found as follows. THE DIESEL CYCLE The Diesel Cycle proceeded the dual combustion cycle. The Diesel cycle is a reasonable approximation of what happens in slow running engines such as large marine diesels. The initial accumulation of fuel and sharp detonation does not occur and the heat input is idealized as a constant pressure process only. Again consider this cycle as being carried out inside a cylinder fitted with a piston. The p-V and T-s cycles diagrams are shown in figure WORKED EXAMPLE An engine using the Diesel Cycle has a compression ratio of 20/1 and a cut off ratio of 2. At the start of the compression stroke the air is at 1 bar and 15oC. Calculate the following. i. The air standard efficiency of the cycle. ii. The maximum temperature in the cycle. iii. The heat input. iv. The net work output. SOLUTION Gas Turbine Power Plants Two simple gas turbines: The Air-Standard Analysis Based on 2 assumptions: · Working fluid is air and it behaves as an ideal gas. · The temperature rise that would actually be brought about by combustion is modeled as being accomplished by heat transfer from an external source (this simplifies the analysis). The Air-Standard Brayton Cycle · Based on the two air-standard analysis assumptions. · The turbine exhaust air is restored to the compressor inlet state by being passed through a heat exchanger which affects Qout to the surroundings The Air-Standard Brayton Cycle Analysis Gas Turbines With Reheat Due to metallurgical considerations, the temperature of gaseous combustion products must be limited. This is done by providing air in excess of the amount required to burn the fuel in the combustor. Therefore, gases leaving the combustor (and the turbine) have sufficient air for further combustion. This is exploited in a multistage turbine with additional combustors between the individual stage Thermodynamics Vapor Power Cycles Example Saturated vapour steam enters the turbine at 8 MPa and saturated liquid leaves the condenser at 0.008 MPa. Find: h, m , Q in (for boiler), Q out (for condenser) if W net =100 MW In actuality, there are irreversibilities in vapour power cycles – most notably those associated with the turbine and pump. Effects of Boiler and Condenser Pressures on the Rankine Cycle Consider the standard IDEAL Rankine Cycle: The area marked in red (1 – b – c – 4 – a – 1) represents the heat transfer into the working fluid per unit mass passing through the boiler, i.e., In the figure above, T in is higher for the cycle with the larger boiler pressure (1’ – 2 ’ – 3 ’ – 4 ’ 1’) than for the lower boiler pressure (1 – 2 – 3 – 4 – 1). Thus as the boiler pressure increases, so does the thermal efficiency. In the figure above, Tout is lower for the cycle with a lower condenser pressure (P < Patm) (cycle 1 – 2” – 3” – 4” – 1) than for the cycle with the higher pressure (P = Patm). Thus as the condenser pressure decreases, the thermal efficiency increases. Improvements to Rankine Cycle It is common practice to ensure that the quality of the steam (or other fluid) at the turbine exit is not less than 0.9 . 1. Superheat After the boiler, further energy is added to the steam in a superheater to bring it to a superheated vapour state (boiler + superheater = “steam generator”). 2. Reheat In a reheat cycle, the steam expands to the condenser in more than one stage. For example, the steam is passed through the 1st stage of a turbine, is reheated in the steam generator to the same T as for the inlet of the 1st stage + passed through a 2nd stage of the turbine to the condenser. More than two stages for a turbine are possible. A reheat cycle allows for taking advantage of the increase thermal efficiency associated with a higher boiler pressure while avoiding low quality steam at the turbine exhaust. Thermodynamics Some Refrigeration Cycles Reversed Carnot Refrigerator and Heat Pump Shown below are the cyclic refrigeration device operating between two constant temperature reservoirs and the T-s diagram for the working fluid when the reversed Carnot cycle is used. Recall that in the Carnot cycle heat transfers take place at constant temperature. If our interest is the cooling load, the cycle is called the Carnot refrigerator. If our interest is the heat load, the cycle is called the Carnot heat pump. The P-h diagram is another convenient diagram often used to illustrate the refrigeration cycle. The Vapor-Compression Refrigeration Cycle The vapor-compression refrigeration cycle has four components: evaporator, compressor, condenser, and expansion (or throttle) valve. The most widely used refrigeration cycle is the vapor-compression refrigeration cycle. In an ideal vapor-compression refrigeration cycle, the refrigerant enters the compressor as a saturated vapor and is cooled to the saturated liquid state in the condenser. It is then throttled to the evaporator pressure and vaporizes as it absorbs heat from the refrigerated space. The ideal vapor-compression cycle consists of four processes. Ideal Vapor-Compression Refrigeration Cycle Process Description 1-2 Isentropic compression 2-3 Constant pressure heat rejection in the condenser 3-4 Throttling in an expansion valve 4-1 Constant pressure heat addition in the evaporator Major Components in Vapour Compression Cycle Refrigeration Cycle: PH diagram Evaporator A diagram of a typical vapor-compression refrigeration cycle is superimposed on a pressure-enthalpy (P-h) chart to demonstrate the function of each component in the system. The pressure-enthalpy chart plots the properties of a refrigerant— refrigerant pressure (vertical axis) versus enthalpy (horizontal axis). The cycle starts with a cool, low-pressure mixture of liquid and vapor refrigerant entering the evaporator where it absorbs heat from the relatively warm air, water, or other fluid that is being cooled. This transfer of heat boils the liquid refrigerant in the evaporator, and this superheated refrigerant vapor is drawn to the compressor. Refrigeration Cycle: PH diagram Compressor The compressor draws in the superheated refrigerant vapor and compresses it to a pressure and temperature high enough that it can reject heat to another fluid. This hot, high-pressure refrigerant vapor then travels to the condenser. Refrigeration Cycle: PH diagram Condenser Within the condenser, heat is transferred from the hot refrigerant vapor to relatively cool ambient air or cooling water. This reduction in the heat content of the refrigerant vapor causes it to desuperheat, condense into liquid, and further subcool before leaving the condenser for the expansion device. Refrigeration Cycle: PH diagram Expansion Device The high-pressure liquid refrigerant flows through the expansion device, causing a large pressure drop that reduces the pressure of the refrigerant to that of the evaporator. This pressure reduction causes a small portion of the liquid to boil off, or flash, cooling the remaining refrigerant to the desired evaporator temperature. The cooled mixture of liquid and vapor refrigerant then enters the evaporator to repeat the cycle. The ordinary household refrigerator is a good example of the application of this cycle. Q L C O PR W Q H W net , in C O PH P net , in h1 h 4 h 2 h1 h2 h3 h 2 h1 Example 11-1 Refrigerant-134a is the working fluid in an ideal compression refrigeration cycle. The refrigerant leaves the evaporator at -20oC and has a condenser pressure of 0.9 MPa. The mass flow rate is 3 kg/min. Find COPR and COPR, Carnot for the same Tmax and Tmin , and the tons of refrigeration. Using the Refrigerant-134a Tables, we have kJ h 238.41 1 C om pressor inlet kg o T1 2 0 C s 0.9456 kJ 1 kg K x1 1.0 kJ C om pressor exit h 2 s 278.23 kg P2 s P2 9 0 0 kP a T 43.79 o C kJ 2s s 2 s s1 0.9456 kg K kJ h 101.61 3 C ondenser exit kg P3 9 0 0 kP a s 0.3738 kJ 3 kg K x 3 0 .0 x 4 0.358 T hrottle exit kJ o T 4 T1 2 0 C s 4 0.4053 kg K h 4 h3 State 1 State 3 State 2 State 4 C O PR QL W net , in m ( h1 h 4 ) m ( h 2 h1 ) (238.41 101.61) (278.23 238.41) h1 h 4 h 2 h1 kJ kg kJ kg 3.44 The tons of refrigeration, often called the cooling load or refrigeration effect, are Q L m ( h1 h 4 ) 3 kg (238.41 101.61) m in kJ kg 1 T on kJ 211 m in 1.94 T on C O PR , C arnot TL TH TL ( 20 273) K (43.79 ( 20)) K 3.97 Another measure of the effectiveness of the refrigeration cycle is how much input power to the compressor, in horsepower, is required for each ton of cooling. The unit conversion is 4.715 hp per ton of cooling. W net , in QL 4.715 C O PR 4.715 hp 3.44 T on 1.37 hp T on Superheating Superheating occurs inside the final length of tubes at which the temperature difference between refrigerant and air is highest Such large temperature difference increases the rate of heat transfer and the refrigerant vapor absorbs much heat. Liquid refrigerant completely evaporated Superheating shifts from the liquid/vapor region to vapor It ensures the refrigerant vapor is completely free liquid before entering the compressor. Heat Pump Systems