PowerPoint - Maxwell at Acadia
advertisement
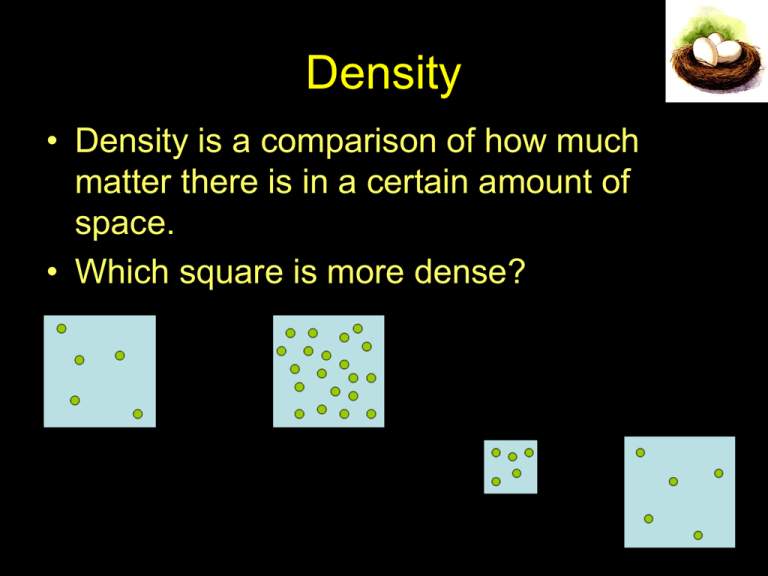
Density • Density is a comparison of how much matter there is in a certain amount of space. • Which square is more dense? Calculating Density • Density = mass volume – solids: d = grams/cubic centimeters (g/cm3) – liquids: d = grams/milliliters (g/ml) ALWAYS REMEMBER THE UNITS! To get the mass just weigh the object Volume Length Height Width • for regular shapes – volume = length x width x height e.g. = 10 cm3 • for irregular shapes – submerge object in a liquid – measure the volume of liquid that is displaced in ml – e.g. key displaced 3 ml of liquid therefore its volume is 3 ml • for liquids – measure directly in ml – e.g. 50 ml Buoyancy and Buoyant Forces When you are swimming in water, there are two forces that work against each other and affect the motion of your body. • the force of gravity is pulling you down • the water is also pushing Bouyancy • Buoyant force, or buoyancy, is the upward force on objects submerged in or floating on fluids. • A buoyant force pushes away from the centre of Earth. Buoyancy • BUOYANCY is the tendency of an object or substance to float Gravity – downward force of gravity pulling on the object Buoyant Force – upward force of fluid pushing on the object Bouyancy • if gravity > buoyant force → object sinks • neutral buoyancy gravity = buoyant force object stays suspended • if gravity < buoyant force → object rises or floats Bouyancy • an object will float if its buoyant force, when fully immersed, is greater than its weight (gravitational force) • it will sink if its weight is greater than the buoyant force • it will float when the buoyant force is equal to its weight (or the force of gravity) Archimede’s Principle This principle explains why some objects float in water and others sink. Salt vs. Fresh water • Seawater (salt water) has a density of 1.03 g/mL and fresh water has a density of 1.00 g/mL. • Therefore, one litre of salt water weighs more than one litre of fresh water. • That is, salt water can support more weight per volume than fresh water, so it is easier to float in salt water. Average Density The average density of an object is the total mass of all substances that make up the object divided by the total volume. • Average density results in objects that would normally sink being able to float. • Examples of technologies that have been developed because of our understanding of density and buoyancy include: 1. Ships Ships can be built of steel because their hollow hull ensures that the average density of the ship is less than that of water. 2. Personal Floatation devices (Life jackets) • Personal flotation devices (ex.Life jackets) are filled with a substance of very low density. • This way, a life jacket lowers a person’s average density, allowing the person to float. 3. Submarines o By allowing water to flow in or out, a submarine can rise or sink in the water. o The submarine floats when its weight is equal to the buoyant force and it sinks when its weight is greater than the buoyant force. 4. Hot Air Balloons When the air inside a hot-air balloon is heated, the air particles: • gain energy and • spread out (forcing some of the particles out of the balloon) The air inside the balloon becomes less dense than the air surrounding Average Density So: • an object will float if its average density is less than the fluid in which it is immersed • an object will sink if its average density is denser than the fluid in which it is immersed • when the object’s density is the same as the medium, an object will neither sink nor float; it is said to be neutrally buoyant. Sink or Float? • wooden boats vs. a water logged stick • metal block vs. metal boats • a sealed, empty plastic bottle vs. a plastic bottle full of water How are Density and Buoyancy Related? How Can Very Dense Objects Float? • Their average density is less than the fluid they float in... • Because they have air holes or are spread out over a large area and filled with air How can dense things float? • the density of steel is 9.0 g/cm3 • the density of water is 1.0 g/ml and the density of sea water is 1.03 g/ml • how can a ship made of steel float? Archimedes discovered that: A ship will float when the weight of the water it displaces equals the weight of the ship. AND Anything will float if it is shaped to displace its own weight of water before it reaches the point where it will submerge. Average Density • average density – the total mass of ALL the substances that make up an object divided by the total volume of the object • humans, fish, ships, submarines, etc can float because of average density – they have lots of air or water spaces inside them Archimedes Principle • Buoyant Force = weight in air – weight in fluid e.g. 7 lbs – 4 lbs = buoyant force of 3 lbs and the weight of the water displaced by the object is also 3 lbs Don’t forget air is also a fluid… • so the principles that apply to having something float in water also apply to having something float in air! Hydrometer • uses buoyancy to measure density directly • calibrated with markings to show density in g/ml, • a denser liquid holds the hydrometer up higher • a less dense liquid allows the hydrometer to sink more