SC3 (f): Relate light emission and the movement of electrons to
advertisement
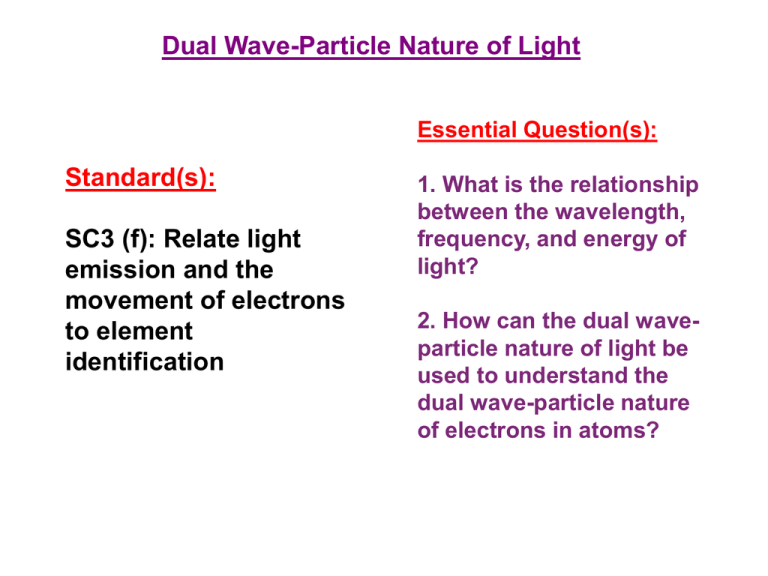
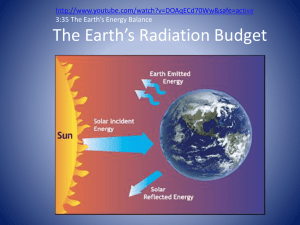
Dual Wave-Particle Nature of Light Essential Question(s): Standard(s): SC3 (f): Relate light emission and the movement of electrons to element identification 1. What is the relationship between the wavelength, frequency, and energy of light? 2. How can the dual waveparticle nature of light be used to understand the dual wave-particle nature of electrons in atoms? Performance Tasks: • Discuss the dual wave-particle nature of light and relate it to the electron. • Calculate the mathematical relationship among the speed, wavelength, and frequency of electromagnetic radiation A. Rutherford’s Atom Electromagnetic Radiation • Energy travels through space by electromagnetic radiation – – – – Gama Rays Ultraviolet Infrared Radio wave X-rays Visible Light Microwaves • The different forms of electromagnetic radiation exhibit the same type of wavelike behavior and travel at the speed of light in a vacuum. B. Energy and Light • Electromagnetic radiation Waves have 3 primary characteristics – Wavelength = lambda ()= distance between 2 consecutive peaks or troughs – Frequency = nu () = number of waves (cycles) per second that pass a given point in space – Since all waves travel at the speed of light, short wavelength radiation must have a high frequency. =c C = speed of light (2.9979*108m/s) And are inversely proportional Units • Wavelength = lambda ()= meters • Frequency = nu () = s-1 or hertz The Nature of Waves Electromagnetic Waves B. Energy and Light • Dual wave-particle nature of light – Wave – Photon – packet of energy B. Energy and Light • Different wavelengths carry different amounts of energy. The Nature of Matter • Matter and energy are not distinct!!! – Max Planck • Question: Is energy continuous? (Transfer of any quantity of energy is possible) • Experiment: studied the radiation bodies emitted by solid bodies heated to incandescence. • Result: matter could absorb or emit any quantity of energy. • Conclusion: energy can be gained or lost only in whole-number multiples of the quantity h (energy is quantized and can only occur in discrete units of size h). Energy has particulate properties Energy is Quantized • E = h – h = Plank’s constant (6.626 * 10 –34 J*s) – = frequency of electromagnetic radiation absorbed or emitted. • Each of these small packets of energy is called a quantum. • A system can transfer energy only in whole quanta • Therefore, energy seems to have particulate properties. Pickle Light The Energy of a Photon • Albert Einstein proposed that electromagnetic radiation is itself quantized. – Hypothesis: electromagnetic radiation can be viewed as a stream of “particles” called photons – Ephoton = h = hc – h = Plank’s constant (6.626 * 10 –34 J*s) – = frequency of electromagnetic radiation absorbed or emitted – is the wavelength of the radiation C. Emission of Energy by Atoms • Atoms can give off light. – They first must receive energy and become excited. – The energy is released in the form of a photon. Photoelectric Effect • Electrons are emitted from the surface of a metal when light strikes it. • Electromagnetic radiation is absorbed by matter only in whole numbers of photons. • In order for an electron to be ejected from a metal surface, the electron must be struck by a single photon possessing at least the minimum energy required to knock the electron loose • The minimum of energy corresponds to the minimum of frequency. Photoelectric Effect – Photon’s frequency is below the minimum, then the electron remains bound to the metal surface – Different metals require different minimum frequencies to exhibit the photoelectric effect. – The KE of the emitted electrons increases linearly with the frequency of the light with a frequency higher than the threshold frequency. The Photoelectric Effect Photoelectric Effect • Electromagnetic radiation is quantized (consists of photons), and the threshold frequency represents the minimum energy required to remove the electron from the metal surface. KEelectron = ½ m2 = h - h0 • The intensity of light is a measure of the number of photons present in a given part of the beam • A greater intensity means that more photons are available to release electrons. Planck & Einstein’s Results: • Energy is quantized. It can occur only in discrete units called quanta. • Electromagnetic radiation and electrons have dual wave-particle nature.