Electrostatics
advertisement

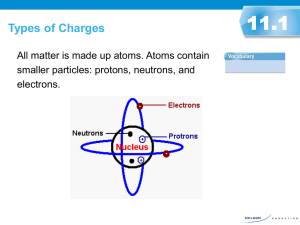
Electrostatics Electrostatics The study of electrical charges that can be collected and held in one place. To understand electricity we must understand the atom. Protons – positive charge, held in the nucleus by the strong nuclear force. Electrons – negative charge, found outside of the nucleus Neutrons – neutral charge, also found in the nucleus. Elementary charge The charge on both a proton and an electron is known as the elementary charge (e). (cover of reference tables) Protons carry a positive elementary charge Electrons carry a negative elementary charge. Charged Particles Most atoms contain an equal number of protons and electrons. Therefore they are electrically neutral. An atom with more protons than electrons will have a positive net charge. An atom with more electrons than protons will have a negative net charge. Obtaining Charges The transfer of electrons to or from an object, causing a deficiency or excess of electrons. An object loses electrons it will be + An object gains electrons it will be – Grounding an object allows for excess electrons be released or more electrons to be gained to achieve a neutral charge. Charges Interacting Two objects that are both positively charged (+) (+), or both negatively charged (-) (-), repel one another. Two objects that have opposite charges respectively, (+) (-), or (-) (+), attract one another. Neutral Objects A positively charged object will attract either a negatively charged object or a neutral object. A negatively charged objects will attract a positively charged object or a neutral object. This is caused by an induced charge…..more to come on that later. Conservation of Charge Your hair and a comb are both neutral. Running the comb through your hair causes electrons to be stripped off of your hair and added to the comb. Your hair’s charge is now _____ The comb’s charge is now ______ The number of electrons lost by your hair is equal to the number of electrons gained by the comb. elost = egained Insulators and Conductors Conductor – material which readily transfers charge. Insulator – material which does not transfer charge easily. Insulators and conductors can be charged by contact. This occurs when there is a transfer of electrons between two objects which are rubbed together. Example: Rubber and fur. Fur has a low affinity for electrons. Rubber has a high affinity for electrons. Electroscope – a device that can detect the presence of an electric charge and the charges sign. (ex. Pith ball) Charging by Conduction Neutral pith ball Positively charged rod moves toward pith ball. Upon contact, the electrons flow from the pith ball into the rod. Now both objects have a net positive charge. Transferring Charge Add the two charges and divide by two to find the final charge. Object A Object B Object C +14e -46e Neutral If A comes in contact with B what will each charge be? A = B= If B comes in contact with C what will each charge be? B = C= If C comes in contact with A what will each charge be? C = A= Charging By Induction Induction – charging an object by bringing it near another charged object. Two neutral conducting spheres The negatively charged PVC pipe repels the electrons in the sphere to one side. Moving the spheres apart, sphere A is left with a positive net charge. Measuring Charge Charge (q) – measured in a unit called the Coulomb (C). The smallest unit of charge called the elementary charge is equal to the charge on a single electron (e). e = -1.6 x 10-19 C A proton has a charge of + 1.6 x 10-19 C ****A charge will always be a multiple of this number since we can’t have ½ of an electron or proton**** Coulomb’s Law Two charged objects may experience motion toward or away from one another. Therefore there must be a force acting on these objects. The electrostatic force is as follows: Fe = k (q1q2) r2 where k is the electrostatic constant = 8.99 x 109 N m2/C2 The electrostatic force is dependant upon the magnitude of the charges as well as the distance between the objects. What equation does this appear similar too? Fe >>Fg Fe always acts on a straight line between objects. Electric force must be a field force since there is no contact between the objects. Practice Example Problem: Find the electrostatic force between these two point charges. The distance between them is 2.3 x 10-3 m. Fe = k (q1q2) r2 Fe = 12760 N Attraction Magnitude and Direction This force, like other forces is a vector. (magnitude and direction) When finding the magnitude of the force, ignore the positive or negative charge. The direction will have to be found by interpreting the charges relations. F1-2 in the previous question is to the left because 1 is attracting 2. Practice #1 How many excess electrons are on a sphere with a charge of -9.20x10-17C? 575 electrons Practice #2 Two charges q1 and q2 are a distance d apart and exert a force F on each other. What will the new force be if: q1 is doubled and q2 is cut in half. F q1 is tripled and q2 is doubled. 6F q2 is cut in half and d is tripled. 1/18 F Practice #3 Two negative charges of -24C each are separated by 6.0cm. What force exists between the charges? 1400N repulsive force Practice #4 Determine the magnitude of the electrostatic force between a proton and an electron that are separated by a distance of 7.5x10-8m 4.1 x 10-14N