Nov18
advertisement
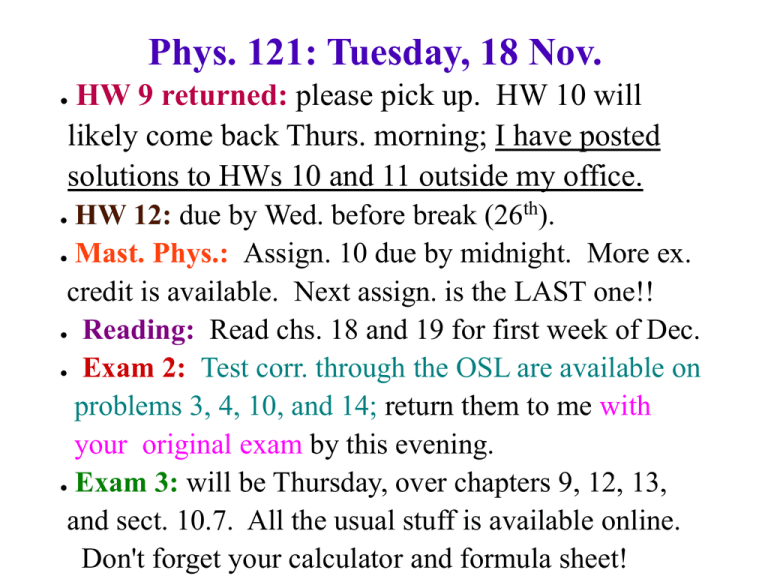
Phys. 121: Tuesday, 18 Nov. HW 9 returned: please pick up. HW 10 will likely come back Thurs. morning; I have posted solutions to HWs 10 and 11 outside my office. ● HW 12: due by Wed. before break (26th). ● Mast. Phys.: Assign. 10 due by midnight. More ex. credit is available. Next assign. is the LAST one!! ● Reading: Read chs. 18 and 19 for first week of Dec. ● Exam 2: Test corr. through the OSL are available on problems 3, 4, 10, and 14; return them to me with your original exam by this evening. ● Exam 3: will be Thursday, over chapters 9, 12, 13, and sect. 10.7. All the usual stuff is available online. Don't forget your calculator and formula sheet! ● Clickers: A mass oscillates on a horizontal spring with period T 2.0 s. If the amplitude of the oscillation is doubled, the new period will be A. 1.0 s. B. 1.4 s. C. 2.0 s. D. 2.8 s. E. 4.0 s Slide 14-51 Notice that ω (and therefore also T and f) is NOT adjustable; it's always the same for a given mass and spring. In contrast, both A and φ depend upon the particular motion (initial conditions). Clickers: A block of mass m oscillates on a horizontal spring with period T 2.0 s. If a second identical block is glued to the top of the first block, the new period will be A. 1.0 s. B. 1.4 s. C. 2.0 s. D. 2.8 s. E. 4.0 s. Clickers: This is the position graph of a mass oscillating on a horizontal spring. What is the phase constant 0? A. /2 rad. B. 0 rad. C. /2 rad. D. rad. E. None of these. QuickCheck 14.2 This is the position graph of a mass oscillating on a horizontal spring. What is the phase constant 0? A. /2 rad. B. 0 rad. C. /2 rad. D. rad. E. None of these. Initial conditions: x=0 vx > 0 Clickers: A block oscillates on a very long horizontal spring. The graph shows the block’s kinetic energy as a function of position. What is the spring constant? A. B. C. D. E. 1 N/m. 2 N/m. 4 N/m. 8 N/m. I have no idea. Internal Energy and Temperature Besides kinetic and potential energy, objects made up of smaller particles (atoms, molecules) can also have internal energy. Internal energy is simply the kinetic energy of the smaller particles bouncing against each other randomly (and not that due to the net motion of the large object). When two such objects touch each other, their molecules can now bounce against each other at the interface, and so internal energy can be transferred. If no net transfer occurs, we say the objects have the same temperature. Temperature and the Zeroth Law of Thermodynamics If internal energy has a net flow from object A to object B when they come into contact, we say that object A has a higher temperature than object B. If there is no net flow, they have the same temperature. Different scales of temperature exist (see next slide). Zeroth Law of Thermodynamics: if objects A and C are at the same temperature, and so are objects B and C, then objects A and B are also at the same temperature. Conversions: T_C = T_K – 273.15 T_F = (9/5) T_C + 32 Clickers: At what temperature do the Fahrenheit and Centigrade temperature scales agree with one another? a) Never b) At 0 degrees c) At 100 degrees d) At -40 degrees e) At -215 degrees Clickers: What is heat? a) A fluid which raises temperature when added to an object (and lowers temp. when removed) b) Potential energy which is bound in molecules c) Potential energy which is pure rotational d) Kinetic energy of the center of mass of large objects, plus its net rotational K.E. e) Kinetic energy whose details are hard to keep track of Clickers: Benjamin Thompson, aka Count Rumford, found (while boring cannon during the American Revolution) that heat kept coming out (and so heat wasn't a fixed liquid). He also... a) was born in America b) invented thermal underwear c) invented the double boiler (for cooking) d) fought for the British during the war e) all of the above Mechanical Equivalent of Heat Heat is internal energy which is being put into or taken out of an object. Changes in heat go with changes in temperature. Because of this, temperature changes can be made into energy changes! The ratio of energy added or taken out (in Joules) to the temperature change (in degrees of any kind) is called the heat capacity of the object. (But, heat capacity increases with object size!) Mechanical Equivalent of Heat (Joule's experiment): Specific Heat c is heat capacity per unit mass, and depends only on what the object is made of... not how large it is! Clickers: Your oven is at 400 degrees F. Which of these objects inside could you safely grab with your hands? • • • • a) A steel frying pan b) A potato c) A thin sheet of aluminum foil d) An angry lobster (who is still alive) that you're trying to bake for dinner Clickers: A cheeseburger with 500 food calories (Cal) has enough energy to give how much mass a speed of 1 m/s? • • • • • a) 1 gram b) 0.5 kg c) 1 kg d) 100 kg e) 4.2 million kg Example: How long should you microwave 330 mL of water at 10˚ C (in a 900 W oven) so that it just reaches the boiling point? Hot and Cold coming to equilibrium: if the two objects are insulated from the surroundings, set the heat (ΔQ) lost by the hot object equal to the heat gained by the cold one, and set the final temperatures to be equal. The heat capacities determine the rest. Clickers: if a hot rock of mass 250 grams is dropped into an equal mass of cold water, whose temperature changes the most? a) The rock b) The water c) They will have equal temperature changes. d) Need more information to tell. Example: A 1.1 kg iron horseshoe at 550˚ C is plunged into a bucket full of 15 kg of water at 20˚ C. Find the final equilibrium temperature. Heat capacity is NOT well defined during a phase change. Notice the constant temperature during the changes! Instead, a fixed amount of heat per mass is needed to make the phase change (and the temperature remains constant during this). It's called the latent heat or the heat of transformation. L depends upon the substance, and whether it's the freezing/melting point (L_f for “fusion”) or the boiling/condensation point (L_v for “vaporization”). Table 17.3 on p. 482 lists a few of these.














