Ideal Gas Law
advertisement

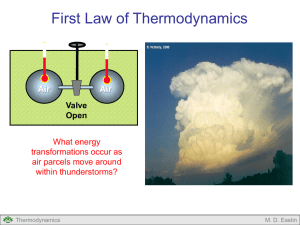
Gas Laws and Equation of State What are the “guts” of air parcels within thunderstorms? Thermodynamics M. D. Eastin Gas Laws and Equation of State Outline: Basic Definitions Kinetic Theory of Gases Early Gas Laws Avogadro’s Hypothesis Ideal Gas Law Thermodynamics M. D. Eastin Basic Definitions Thermodynamics: The study of equilibrium states of a system System: Any specific sample of matter (Examples: an “air parcel” or the air within a balloon) • Open system: Exchanges matter and energy with its surroundings. (Example: a balloon with a lot of holes) • Closed system: Does not exchange matter with its surroundings, but can exchange energy. (Example: a balloon without insulation) • Isolated System: Does not exchange matter or energy with its surroundings. (Example: a balloon with insulation) Note: In meteorology we assume most systems are closed Thermodynamics M. D. Eastin Basic Definitions Thermodynamics: State: The study of equilibrium states of a system Defines the total volume (V), pressure (p), and temperature (T) of each constituent composing a system at any given time If the system is homogeneous (contains only one component), then only the V, p, T values are needed. If the system is heterogeneous (contains multiple components), then the concentrations of the different components as well as the V, p, T are needed. Note: Our atmosphere is a heterogeneous system: Thermodynamics M. D. Eastin Basic Definitions Thermodynamics: Equillibrium: The study of equilibrium states of a system Defines as a state in which a system’s properties do not change as long as the properties of the surroundings remain unchanged Example: Surroundings: V2, P2, T2 What would happen if the air pressure in the surroundings decreases? Closed System V1, P1, T1 Equilibrium: P1 = P2 and T1 = T2 Thermodynamics What would happen if the air temperature in the surroundings decreases? M. D. Eastin Basic Definitions Transformation: When a system changes from an initial state to a new final state Symbolically: i→f Reversible transformation: Any transformation that can be reversed and arrive back at the initial state ( i → f → i ) Occur when the external surroundings change very slowly so the system has time to adjust to new equilibrium states along the way Irreversible transformation: Any transformation that can not be reversed and arrive back at its original state. Occur when surroundings or the system experiences a rapid change Thermodynamics M. D. Eastin Basic Definitions Transformation: When a system changes from an initial state to a new final state Isothermal transformation: Any transformation that occurs without a change in temperature Isochoric transformation: Any transformation that occurs without a change in volume Isobaric transformation: Any transformation that occurs without a change in pressure Adiabatic transformation: Any transformation that occurs without exchanging heat with the environment Note: Adiabatic transformations are not isothermal Thermodynamics M. D. Eastin Basic Definitions Energy: The total internal energy of a system (U) is the sum of the kinetic energy and potential energy of all the particle’s that compose the system. Each particle is moving in some random direction (kinetic energy) Each particle is being pulled down by gravity (potential energy) Thermodynamics M. D. Eastin Kinetic Theory of Gases Basic Idea: We have a closed system containing N molecules moving in random directions and random speeds: The mean kinetic energy of all molecules is the system temperature (T) The mean force exerted on the system’s edges due to particle collisions with the edge is the system pressure (p). The three-dimensional space occupied by the moving molecules is the system volume (V) Thermodynamics M. D. Eastin Kinetic Theory of Gases Basic Idea: By applying some simple laws of physics (see Chapter 2 in your text), a simple equation that combines the three state variables into a single equation can be obtained: pV NkT where: p = pressure V = volume N = Number of molecules k = Boltzmann’s constant T = temperature Note: This is one form of the ideal gas law This equation was obtain from the results of numerous laboratory experiments during the 18th and 19th centuries. Lets examine some of those early results… Thermodynamics M. D. Eastin Early Gas Laws Gay-Lussac Law #1: When pressure (p) is constant: V1 T1 V2 T2 Gay-Lussac Law #2: When volume (V) is held constant: p1 T1 p2 T2 Boyles Law: When temperature (T) is held constant: p1V1 p2V2 Note: The subscripts refer to two different system states Thermodynamics M. D. Eastin Early Gas Laws Example: Assume a hot air balloon is anchored to the ground and the air within occupies V = 10 m3 at p = 1000 mb at T = 300 K. The burners at the balloon based are turned on and the air heats up to T = 320 K. Assume the balloon volume remains constant. What is the new pressure inside the balloon? Answer: Apply the Gay-Lussac Law #2: T1 = 300 K p1 = 1000 mb T2 = 320 K Solve the equation for p2 p1 T1 p2 T2 p1T2 p2 T1 Plug in the values → p2 = 937.5 mb Thermodynamics M. D. Eastin Avogadro’s Hypothesis (Law) Avogadro’s Number: The number of atoms in 12 g of carbon Called a mole → 1 mole = 6.022 ×1023 Avogadro’s Hypothesis: One mole of any gas at constant temperature and pressure occupies the same volume If we increase the number of molecules (or mass) of the gas at a constant temperature and pressure, then the volume of the system increases. Thermodynamics M. D. Eastin Ideal Gas Law • If we combine Boyles Law and Gay-Lussac’s second law we obtain: p1V1 p2V2 T1 T2 which implies that if we change either p, V, or T, the value of pV/T remains constant. Thus we can re-write the equation above as: pV AT where A is a constant that depends on the type and amount of the gas. Note: The dependence on the amount (or mass) of the gas is a result of Avogadro’s Hypothesis Thermodynamics M. D. Eastin Ideal Gas Law • Since A is a function of both gas type and its mass, we can define A as: A mR where: m = total mass of the gas R = constant independent of mass, but dependent on gas type (will change for different gas types: oxygen vs. hydrogen) and re-write our equation as: pV mRT Thermodynamics Ideal Gas Law M. D. Eastin Ideal Gas Law • Since density (ρ) is defined as the mass (m) per unit volume (V): m V we could re-write our equation as: p RT pV mRT → Ideal Gas Law • Also, since we can define a specific volume (α): 1 we could re-write our equation as: pV mRT → Thermodynamics p RT Ideal Gas Law M. D. Eastin Ideal Gas Law • Also, if we let M equal the molecular weight of the gas, then the number of moles of that gas is defined as: m n M where: n = number of moles m = total mass of the gas M = molecular weight of the gas We could also re-write our equation as: pV mRT → Thermodynamics pV nMRT Ideal Gas Law M. D. Eastin Ideal Gas Law • Both M and R are constants and each are dependent on gas type • However, their product results in a constant that is independent of gas type, and is thus called the universal gas constant (R*): R * MR where: R* = 8.3143 J K-1 mol-1 We can then rewrite our equation as: * → pV nR T pV nMRT Thermodynamics Ideal Gas Law M. D. Eastin Ideal Gas Law Formulations of the Ideal Gas Law: pV NkT pV nMRT pV nR*T pV mRT p RT → where: Thermodynamics p RT ← p = pressure V = volume T = temperature N = number or molecules k = Boltzmann’s constant (1.38 ×10-23) n = number of moles M = molecular weight of a gas particle R = gas constant dependent on gas type m = total mass ρ = density (m/V) α = specific volume (1/ρ) R* = universal gas constant (8.3143 J K-1 mol-1) M. D. Eastin Ideal Gas Law: Earth’s Atmosphere Ideal Gas Law for Earth’s Dry Atmosphere: • We need to find the gas constant (R) valid for our atmosphere, which contains multiple gases in various percentages: R* R M → R* Rd M • We will neglect water vapor, and find the gas constant for our dry atmosphere Gas Molecular Weight Nitrogen (N2) 28.016 Oxygen (O2) 32.000 Argon (Ar) 39.940 Carbon Dioxide (CO2) 44.010 Thermodynamics Percentage by mass 75.51% 23.14% 1.30% 0.05% M. D. Eastin Ideal Gas Law: Earth’s Atmosphere Ideal Gas Law for Earth’s Dry Atmosphere: • Thus, we need to find the mean molecular weight of our atmosphere: Dalton’s Law: M K mi K mi i 1 Mi i 1 See Example 3.1 (on page 21-22) of your text for details M 28.97 g / mol Rd 287 J / kgK Thermodynamics M. D. Eastin Ideal Gas Law: Earth’s Atmosphere Be careful about your interpretation: • The ideal gas law relates three variables (p, ρ or V, and T) • Thus, any change in one does not automatically produce a change in another. • If we differentiate the ideal gas law: p Rd T dp Rd dT Rd Td • A change in pressure (dp) can lead to either a change in temperature (dT) or a change in density (dρ), or both. Let’s see a couple examples… Thermodynamics M. D. Eastin Ideal Gas Law: Earth’s Atmosphere Be careful about your interpretation: Example #1: Assume you are in Charlotte getting ready to board an airline flight to Denver. You have a soft drink bottle that is filled with air at p = 1000 mb, T = 300 K, and ρ = 1.00 kg/m3. Before you boarded the plane, you sealed the bottle tightly. Once you arrived at Denver you noticed the bottle was partially collapsed. You know the temperature in the bottle remained unchanged, but the pressure dropped to p = 850 mb. What was the new air density? dp RdT RTd Can you solve this problem? If “yes”, then do so… (pay attention to units) Thermodynamics M. D. Eastin Ideal Gas Law: Earth’s Atmosphere Be careful about your interpretation: Example #2: Assume you are in Charlotte getting ready to board an airline flight to Denver. You have a soft drink bottle that is filled with air at p = 1000 mb, T = 300 K, and ρ = 1.00 kg/m3. Before you boarded the plane, you sealed the bottle tightly. Once you arrived at Denver you noticed the bottle was partially collapsed. You did not know the bottle temperature but the pressure dropped to p = 850 mb. What was the new air density? dp RdT RTd Can you solve this problem? If “yes”, then do so… (pay attention to units) Thermodynamics M. D. Eastin Gas Laws and Equation of State Summary: • Basic Definitions: • Equilibrium, System, State • Reversible, Irreversible, Isobaric, Isothermal, Isochoric, Adiabatic • Kinetic Theory of Gases (know the basic idea) • Early Gas Laws: • Gay-Lussac Laws • Boyles Law • Avogadro’s Hypothesis (know the basic idea) • Ideal Gas Law: • Basic Derivation • Various Forms • Universal Gas Constant • Dalton’s Law for (mean molecular weights) Thermodynamics M. D. Eastin References Petty, G. W., 2008: A First Course in Atmospheric Thermodynamics, Sundog Publishing, 336 pp. Tsonis, A. A., 2007: An Introduction to Atmospheric Thermodynamics, Cambridge Press, 197 pp. Wallace, J. M., and P. V. Hobbs, 1977: Atmospheric Science: An Introductory Survey, Academic Press, New York, 467 pp. Thermodynamics M. D. Eastin