Clausius-Clapeyron Equation
advertisement
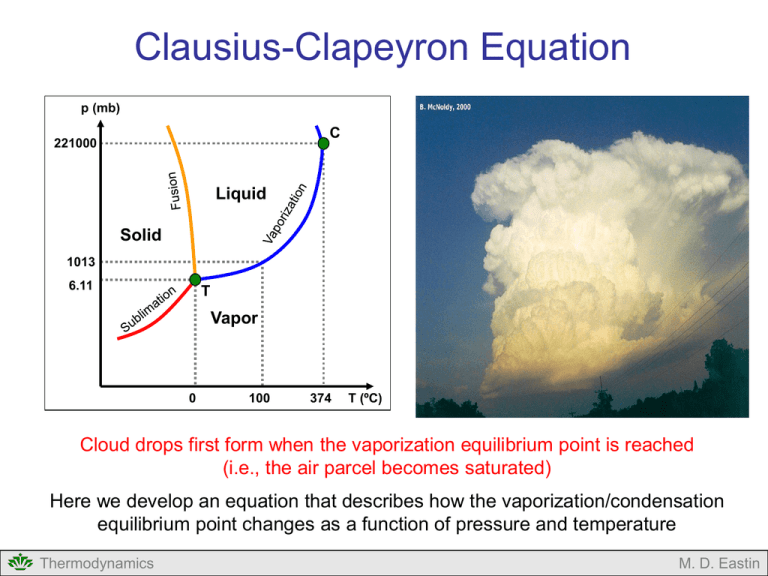
Clausius-Clapeyron Equation p (mb) C 221000 Liquid Solid 1013 6.11 T Vapor 0 100 374 T (ºC) Cloud drops first form when the vaporization equilibrium point is reached (i.e., the air parcel becomes saturated) Here we develop an equation that describes how the vaporization/condensation equilibrium point changes as a function of pressure and temperature Thermodynamics M. D. Eastin Clausius-Clapeyron Equation Outline: Review of Water Phases Review of Latent Heats Changes to our Notation Clausius-Clapeyron Equation Basic Idea Derivation Applications Equilibrium with respect to Ice Applications Thermodynamics M. D. Eastin Review of Water Phases Homogeneous Systems (single phase): Gas Phase (water vapor): • Behaves like an ideal gas • Can apply the first and second laws p v ρ v R vTv Liquid Phase (liquid water): • Does not behave like an ideal gas • Can apply the first and second laws Solid Phase (ice): • Does not behave like an ideal gas • Can apply the first and second laws dq cvdT pd dq rev ds T Thermodynamics M. D. Eastin Review of Water Phases Heterogeneous Systems (multiple phases): Liquid Water and Vapor: • Equilibrium state • Saturation • Vaporization / Condensation • Does not behave like an ideal gas • Can apply the first and second laws Equilibrium States for Water (function of temperature and pressure) p (mb) C 221000 Liquid Solid pv, Tv 1013 6.11 T pv pw Vapor Tv Tw pw, Tw Thermodynamics 0 100 374 T (ºC) M. D. Eastin Review of Water Phases Equilibrium Phase Changes: Vapor → Liquid Water (Condensation): • Equilibrium state (saturation) • Does not behave like an ideal gas • Isobaric • Isothermal • Volume changes pv pw P (mb) Liquid Tv Tw C A B C 221,000 C Solid 6.11 B Liquid and Vapor Solid and Vapor Tc = 374ºC A Vapor T1 T Tt = 0ºC V Thermodynamics M. D. Eastin Review of Latent Heats Equilibrium Phase Changes: • Heat absorbed (or given away) during an isobaric and isothermal phase change L dQ constant P (mb) Liquid C 221,000 Tc = 374ºC L • From the forming or breaking of molecular bonds that hold water molecules together in its different phases • Latent heats are weak function of temperature Vapor L Solid T1 T 6.11 L Tt = 0ºC V Values for lv, lf, and ls are given in Table A.3 of the Appendix Thermodynamics M. D. Eastin Changes to Notation Water vapor pressure: • We will now use (e) to represent the pressure of water in its vapor phase (called the vapor pressure) • Allows one to easily distinguish between pressure of dry air (p) and the pressure of water vapor (e) Temperature subscripts: Ideal Gas Law for Water Vapor p v ρ v R vTv e ρ v R vT • We will drop all subscripts to water and dry air temperatures since we will assume the heterogeneous system is always in equilibrium T Tv Tw Ti Thermodynamics M. D. Eastin Changes to Notation Water vapor pressure at Saturation: • Since the equilibrium (saturation) states are very important, we need to distinguish regular vapor pressure from the equilibrium vapor pressures e = vapor pressure (regular) esw = saturation vapor pressure with respect to liquid water esi = saturation vapor pressure with respect to ice Thermodynamics M. D. Eastin Clausius-Clapeyron Equation Who are these people? Rudolf Clausius 1822-1888 German Mathematician / Physicist Benoit Paul Emile Clapeyron 1799-1864 French Engineer / Physicist “Discovered” the Second Law Introduced the concept of entropy Expanded on Carnot’s work Thermodynamics M. D. Eastin Clausius-Clapeyron Equation Basic Idea: p (mb) • Provides the mathematical relationship (i.e., the equation) that describes any equilibrium state of water as a function of temperature and pressure. • Accounts for phase changes at each equilibrium state (each temperature) C 221000 Liquid Solid 1013 6.11 T Vapor P (mb) Vapor esw 100 374 T (ºC) T Liquid Liquid and Vapor Thermodynamics 0 Sections of the P-V and P-T diagrams for which the Clausius-Clapeyron equation is derived in the following slides V M. D. Eastin Clausius-Clapeyron Equation Mathematical Derivation: Assumption: Our system consists of liquid water in equilibrium with water vapor (at saturation) Isothermal process Adiabatic process B C esw1 esw2 T1 A Volume D T2 Saturation vapor pressure Saturation vapor pressure • We will return to the Carnot Cycle… B, C esw1 A, D esw2 T2 T1 Temperature Thermodynamics M. D. Eastin Clausius-Clapeyron Equation Mathematical Derivation: • Recall for the Carnot Cycle: Q1 Q2 T1 T2 Q1 T1 where: Q1 > 0 and Q2 < 0 • If we re-arrange and substitute: Saturation vapor pressure WNET Q1 Q2 Isothermal process Adiabatic process Q1 B C esw1 T1 WNET esw2 A Q2 D T2 Volume Q1 WNET T1 T1 - T2 Thermodynamics M. D. Eastin Clausius-Clapeyron Equation Mathematical Derivation: Recall: Q1 WNET T1 T1 - T2 • During phase changes, Q = L • Since we are specifically working with vaporization in this example, Q1 Lv T1 T T1 T2 dT Saturation vapor pressure • Also, let: Isothermal process Adiabatic process Q1 B C esw1 T1 WNET esw2 A Q2 D T2 Volume Thermodynamics M. D. Eastin Clausius-Clapeyron Equation Mathematical Derivation: Recall: Q1 WNET T1 T1 - T2 • The net work is equivalent to the area enclosed by the cycle: WNET dV dp • The change in pressure is: • The change in volume of our system at each temperature (T1 and T2) is: dV α v α w dm where: αv = specific volume of vapor αw = specific volume of liquid Saturation vapor pressure desw esw1 esw2 Isothermal process Adiabatic process Q1 B C esw1 T1 WNET esw2 A Q2 D T2 Volume dm = total mass converted from vapor to liquid Thermodynamics M. D. Eastin Clausius-Clapeyron Equation Mathematical Derivation: • We then make all the substitutions into our Carnot Cycle equation: L v α v α w dm de sw T dT • We can re-arrange and use the definition of specific latent heat of vaporization (lv = Lv /dm) to obtain: de sw lv dT Tα v α w Clausius-Clapeyron Equation for the equilibrium vapor pressure with respect to liquid water Thermodynamics Saturation vapor pressure Q1 WNET T1 T1 - T2 B, C esw1 A, D esw2 T2 T1 Temperature M. D. Eastin Clausius-Clapeyron Equation General Form: • Relates the equilibrium pressure between two phases to the temperature of the heterogeneous system Equilibrium States for Water (function of temperature and pressure) p (mb) dp s l dT TΔ where: T = l = dps = dT = Δα = Temperature of the system Latent heat for given phase change Change in system pressure at saturation Change in system temperature Change in specific volumes between the two phases C 221000 Liquid Solid 1013 6.11 T Vapor 0 Thermodynamics 100 374 T (ºC) M. D. Eastin Clausius-Clapeyron Equation Application: Saturation vapor pressure for a given temperature Starting with: de sw lv dT Tα v α w Assume: α v α w [valid in the atmosphere] and using: esw α v R v T [Ideal gas law for the water vapor] We get: de sw lv dT esw R v T2 If we integrate this from some reference point (e.g. the triple point: es0, T0) to some arbitrary point (esw, T) along the curve assuming lv is constant: esw es0 Thermodynamics de sw lv esw Rv dT T0 T 2 T M. D. Eastin Clausius-Clapeyron Equation Application: Saturation vapor pressure for a given temperature esw es0 de sw l v esw Rv dT T0 T 2 T After integration we obtain: esw lv 1 1 ln es0 R v T0 T After some algebra and substitution for es0 = 6.11 mb and T0 = 273.15 K we get: lv 1 1 esw (mb) 6.11 exp R v 273.15 T (K) Thermodynamics M. D. Eastin Clausius-Clapeyron Equation Application: Saturation vapor pressure for a given temperature lv 1 1 esw (mb) 6.11 exp R v 273.15 T (K) A more accurate form of the above equation can be obtained when we do not assume lv is constant (recall lv is a function of temperature). See your book for the derivation of this more accurate form: 6808 esw (mb) 6.11 exp53.49 5.09lnT ( K ) T (K ) Thermodynamics M. D. Eastin Clausius-Clapeyron Equation Application: Saturation vapor pressure for a given temperature 6808 esw (mb) 6.11 exp53.49 5.09lnT ( K ) T (K ) What is the saturation vapor pressure with respect to water at 25ºC? T = 298.15 K esw = 32 mb What is the saturation vapor pressure with respect to water at 100ºC? T = 373.15 K Boiling point esw = 1005 mb Thermodynamics M. D. Eastin Clausius-Clapeyron Equation Application: Boiling Point of Water de sw lv dT Tα v α w At typical atmospheric conditions near the boiling point: T = 100ºC = 373 K lv = 2.26 ×106 J kg-1 αv = 1.673 m3 kg-1 αw = 0.00104 m3 kg-1 de sw 36.21 mb K 1 dT This equation describes the change in boiling point temperature (T) as a function of atmospheric pressure when the saturated with respect to water (esw) Thermodynamics M. D. Eastin Clausius-Clapeyron Equation Application: Boiling Point of Water What would the boiling point temperature be on the top of Mount Mitchell if the air pressure was 750mb? • From the previous slide we know the boiling point at ~1005 mb is 100ºC de sw 36.21 mb K 1 dT • Let this be our reference point: esw esw ref 36.21 mb K 1 T Tref Tref = 100ºC = 373.15 K esw-ref = 1005 mb • Let esw and T represent the values on Mt. Mitchell: T esw esw ref 36.21 Tref esw = 750 mb T = 366.11 K T = 93ºC Thermodynamics (boiling point temperature on Mt. Mitchell) M. D. Eastin Clausius-Clapeyron Equation Equilibrium with respect to Ice: • We will know examine the equilibrium vapor pressure for a heterogeneous system containing vapor and ice p (mb) C 221000 Liquid P (mb) Solid 1013 6.11 Liquid C T Vapor Vapor Solid 0 Thermodynamics 374 T (ºC) T 6.11 esi 100 B A T V M. D. Eastin Clausius-Clapeyron Equation Equilibrium with respect to Ice: • Return to our “general form” of the Clausius-Clapeyron equation p (mb) de s l dT T • Make the appropriate substitution for the two phases (vapor and ice) de si ls dT Tα v α i C 221000 Liquid Solid 1013 6.11 T Vapor 0 100 374 T (ºC) Clausius-Clapeyron Equation for the equilibrium vapor pressure with respect to ice Thermodynamics M. D. Eastin Clausius-Clapeyron Equation Application: Saturation vapor pressure of ice for a given temperature Following the same logic as before, we can derive the following equation for saturation with respect to ice ls 1 1 esi (mb) 6.11 exp R v 273.15 T (K) A more accurate form of the above equation can be obtained when we do not assume ls is constant (recall ls is a function of temperature). See your book for the derivation of this more accurate form: 6293 esi (mb) 6.11 exp26.16 0.555lnT ( K ) T (K ) Thermodynamics M. D. Eastin Clausius-Clapeyron Equation Application: Melting Point of Water • Return to the “general form” of the Clausius-Clapeyron equation and make the appropriate substitutions for our two phases (liquid water and ice) dp wi lf dT Tα w αi At typical atmospheric conditions near the melting point: T = 0ºC = 273 K lf = 0.334 ×106 J kg-1 αw = 1.00013 × 10-3 m3 kg-1 αi = 1.0907 × 10-3 m3 kg-1 dp wi 135,038 mb K 1 dT This equation describes the change in melting point temperature (T) as a function of pressure when liquid water is saturated with respect to ice (pwi) Thermodynamics M. D. Eastin Clausius-Clapeyron Equation Summary: • Review of Water Phases • Review of Latent Heats • Changes to our Notation • Clausius-Clapeyron Equation • Basic Idea • Derivation • Applications • Equilibrium with respect to Ice • Applications Thermodynamics M. D. Eastin References Petty, G. W., 2008: A First Course in Atmospheric Thermodynamics, Sundog Publishing, 336 pp. Tsonis, A. A., 2007: An Introduction to Atmospheric Thermodynamics, Cambridge Press, 197 pp. Wallace, J. M., and P. V. Hobbs, 1977: Atmospheric Science: An Introductory Survey, Academic Press, New York, 467 pp. Thermodynamics M. D. Eastin








